Abstract
Specific heat capacity is the measure of the heat energy
In physics, energy is a scalar physical quantity that describes the amount of Work_ that can be performed by a force. Energy is an attribute of objects and systems that is subject to a conservation law required to increase the temperature
In physics, the temperature is a physical property of a Physical system that underlies the common notions of hot and cold; something that feels hotter generally has the greater temperature of a substance by a certain temperature interval
Celsius is a temperature scale that is named after the Swedish astronomer Anders Celsius, who developed a similar temperature scale two years before his death. The SI units for specific heat capacity are either joule per mole-kelvin (J mol–1 K–1) or joule per gram-kelvin (J g–1 K–1). Joseph Black, a Scottish physicist is the man who carried out heat measurements and employed the phrase “capacity for heat”.
He discovered that additional heat energy was needed to raise the temperature of matter with high specific heat capacity compared with those substances with low specific heat capacity.
Introduction
In this experiment, the principle of specific heat capacity of an ideal gas at constant volume (Cv) and constant pressure (Cp) to determine Heat capacity ratio is abbreviated as gamma, γ. The study of the specific heat capacity of gas gives an insight into the structure of the gaseous molecule. Specificity heat capacity of gas is affected by the degree of freedom of a gas that is it depends on whether it is monatomic or diatomic.
Theoretical Background of the experiment
An audio frequency signal generator of low output impendence was employed to drive exciter coils. An interaction between the magnetic field and the Alnico magnet produces an alternating force that is experienced by the piston. As a result of these interactions, there is the fluctuation of air pressure which is then transmitted to the electric-microphone that is set at end bungs of the tube of glass. Then this signal is displayed on a Cathode Ray Tube (C.R.T+) from which the amplitude of the waveform at a given resonance frequency can be determined. To obtain a sinusoidal graph on C.R.T, the smallest amplitude of drive signal.
Experimental procedure
Clean apparatus was set up as shown in the figure below. The piston was dislodged from its equilibrium position by an alternating force and the signal produced was measured by C.R.T. Then the frequency of the exciter coils current was varied and resonance frequencies (f0, f1, f2) and amplitude of the waveform was recorded at constant volume of the ideal gas. Amplitude was repeated three times and average amplitude was used to draw the graph. Mass (M), volume (V), pressure (P), and cross-section area (A) of the matter were taken at a constant temperature. Based on these results, graphs were drawn and the heat capacity ratio (gamma) calculate.
Results
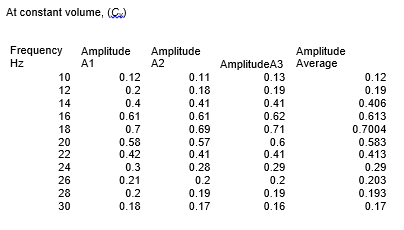
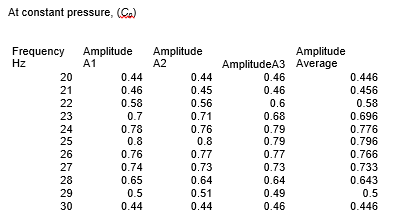
Mass= 7.2 × 10-3 kg cross- section area=1.65×10-4 m2, volume=4.13×10-5 m3
Discussion and Conclusion
From the results, the ratio between the specific heat capacity of an ideal gas at constant volume and constant pressure, γ, was found to be 1.28 at one atmospheric pressure. It was discovered that the constant pressure heat capacity is greater compared to the constant volume for a gas. This is because extra heat is needed for the expansion of gas at constant pressure instead of all being utilized as internal thermal energy.
In this experiment, the heat capacity ratio for air was found to be 1.28 while the known standard is 1.40. An experimental error occurred while Mass, volume, pressure, cross-section area, and resonance frequency were being measured. The percentage error is 8.57% which is within an acceptable range. The objective of the experiment was achieved.