Fuel Cell Vehicles: Status 2007 (2007) was written by Rittmar von Helmolt and Ulrich Eberie, both employees at GM Fuel Cell Activities at Ruesselsheim, Germany. The paper details the developments that have been made towards the use of Hydrogen to propel vehicles.
Rittmar von Helmolt is a scientist and inventor, according to patent websites such as freshpatents.com and patentdocs.org, he has applied for several patents regarding his inventions, most of which relate to motor vehicle mechanics. He has also written several scientific articles and co-authored others, the articles center on motor vehicle mechanics and alternative fuel. Similarly, Dr Ulrich Eberle is a researcher and has written several articles for major scientific journals such as Energy Environ. Sc. and Journal of Power Sources. He is also a scientist and his work at GM Corporation is a testament to his professionalism.
The two authors’ contribution to the paper is valuable as it gives the view of professionals in fuel cell technology, this gives the paper a professional touch and hence makes it a credible source of information for academic purposes. The authors write on a topic they are well versed on. The paper was written in 2007 and this fits the widely accepted 5-year range within which scientific information remains valid. This implies that the information in the paper is up to date and suitable for use as an academic source. Besides, it is written in a scientific journal and thus the authors have a specific target.
The authors refer to several projects being undertaken by major vehicle manufacturing companies such as Opel and Honda. At the end of the paper, von Helmolt and Eberie give a list of references that show the scope of research they undertook in writing the paper, most of these references are drawn from peer reviewed journals and reports from multi-national corporations, hence their credibility.
Article Summary
The article is divided into 7 chapters. In Chapter 1, the authors give an introduction on the use of Hydrogen as fuel and the factors that have contributed to the invention, i.e. environmental concern and rising fuel costs. They introduce the audience to the science behind the fuel cell in chapter 2, giving a systematic process from the production of Hydrogen to its conversion to electricity, and the subsequent conversion of excess Hydrogen atoms to water.
The next three chapters touch on fuel (Hydrogen) storage options and safety features in the fuel tank and fuel cell. Chapter 6 gives the three phases in the development of Hydrogen as an alternative fuel by GM Corporation: Phase 1 ends in 2010, Phase 2 begins from 2010 and ends in 2015, while Phase 3 starts in 2015. They conclude the paper in Chapter 7 by giving their perspective on the use of Hydrogen for powering vehicles, the challenges, advantages, and the costs involved (von Helmolt and Eberie, 2007, pp. 842). I chose to focus on Chapter 2: Fuel cell system, as it gives a detailed analysis of what goes on inside the fuel cell.
Hydrogen Powered Cars
Due to the rising costs of gasoline and environmental concerns, the need for the use of natural, clean and renewable energy is long overdue. Automobiles are one of the leading sources of pollution, hence the need to find alternative sources of energy for running them. It is for this reason that scientists have been undertaking experiments on the use of Hydrogen as a fuel to run motor vehicles. Hydrogen gas fuel produces electricity, or the power to run motor vehicles, through an electrochemical reaction. Unlike gasoline that produces Carbon dioxide when used as fuel, the only by-product obtained from this process is clean water. Besides, hydrogen is easily available from air and water and this lowers the cost of using it as a fuel.
Hydrogen Cell Fuel
The core of a hydrogen-powered vehicle lies in the fuel cell that facilitates the production of electricity for use as a source of kinetic energy. Hydrogen gas can be obtained through catalytic splitting of a water molecule: this is done by passing electricity through water. Splitting of a single water molecule gives two Hydrogen atoms and one Oxygen atom as shown below:
H2O → O– + 2H+
The authors take us through a systematic process that produces electricity as sketched below:
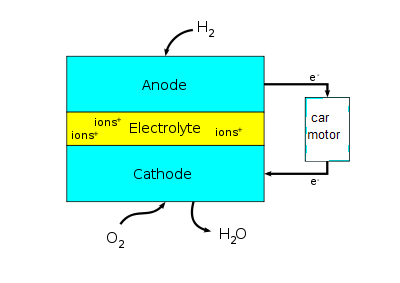
Hydrogen gas enters the fuel cell through an inlet on the anode end and is mixed with a catalyst, this splits the diatomic Hydrogen molecules into electrons and protons. These electrons then flow through the system to produce electricity. Electricity that has been produced is directed to the car’s electric drive motor and converted to kinetic energy. Excess electricity is stored in a back-up battery used when the car runs out of fuel, a Lithium-ion battery is frequently used for this purpose. Protons go through a polymer electrolyte membrane to be reacted with Oxygen.
Oxygen gas enters the fuel cell on the cathode end and reacts with Hydrogen protons and excess electrons to form water as shown below:
O– + 2H+ → H2O
A Hydrogen fuel cell consists of a thin membrane encased between two electrode layers, these, in turn, are placed between two divider layers. A fuel cell consists of several layered cells linked together in series, the number of these cells depends on the type of car, for example, SUVs have more cells than light saloon cars.
Hydrogen gas is continuously pumped into the fuel cell from a tank placed at the back of the vehicle. Similar to regular gasoline engines, the fuel cell has various safety features. For example, the engine automatically shuts off in case of a fuel leak and has a system that cuts off the flow of fuel and electricity in case of a collision. The fuel cell has a safety valve to prevent reverse flow of the fuel, prevent contamination of fuel in the tank, and ensures that gas pressure in the tank remains constant.
Conclusion
Fuel Cell Vehicles: Status 2007 provides useful information towards the use of Hydrogen as a fuel. However, to obtain further information regarding this topic, I may use other sources to expand onto what the two authors have presented in the article, or to see how other authors treat the same topic. Hence, I may refer to Fuel cells: from fundamentals to applications (2006) by Supramaniam Srinivasan.
Reference
Von Helmolt, R., and Eberie, U. (2007). Fuel Cell Vehicles: Status 2007. Journal of Power Sources, Volume 165, Issue 2. 833-843.