Introduction
Green chemistry can be described as the abolition of the use or production of harmful substances in the creation, making, and use of chemical commodities. Green chemistry has been in effect for approximately 20 years. Numerous economic and ecological benefits are the main drivers of the adoption of green chemistry. The international marketplace for green chemistry is expected to expand exponentially in the coming years and is expected to reach $98.5 billion by 2020. Green chemistry lowers operating costs because of the generation of small quantities of waste, which in turn reduces environmental compliance costs.
Low waste production eradicates the need for costly waste treatment processes. Solvents are commonly used materials in industrial and domestic processes. Some of the industrial uses of solvents include acting as media for chemical reactions in the production of different chemicals. Solvents facilitate the purification and separation of substances. Solvents also form a part of everyday consumer products.
However, it is reported that reduced solvent use alongside a few processing stages enhances the efficiency of the manufacturing process, thus lowering material and energy costs. This paper discusses various consumer products that contain non-aqueous solvents and the potential long-term environmental impact of these solvents. Feasible benign alternatives to these solvents are also provided.
Consumer Products with Non-Aqueous Solvents and Their Long-Term Environmental Effects
Non-aqueous solvents include substances other than water that are used as media for chemical reactions. These solvents may be organic or inorganic substances. Examples of non-aqueous organic solvents include carbon tetrachloride, benzene, acetone, ether among many others. On the other hand, inorganic non-aqueous solvents include liquid sulfur dioxide, liquid ammonia, sulfuric acid among others.
Most of these commonly used solvents pose serious environmental concerns in three main areas: the source and production of the solvent, its physical and chemical attributes during use, and their disposal. This section focuses on three consumer products that contain non-aqueous solvents and their long-term environmental impact. The selected consumer products include detergents containing linear alkyl benzene, spot removers with carbon tetrafluoride, and soil fumigants containing nitromethane. The chemical structures of these three non-aqueous solvents are provided in Figure 1.
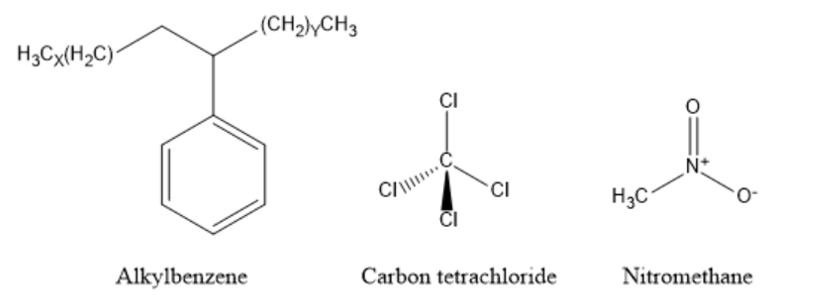
Detergents Containing Linear Alkyl Benzene
A detergent is a substance that consists of a surfactant or a blend of surfactants with cleaning power in dilute solutions. Linear alkyl benzene (LAB) is a commonly used surfactant in the manufacture of detergents. It is often used in the form of alkylbenzenesulfonate, which is referred to as linear alkyl sulfonate (LAS). The use of LAS is preferred due to its high foaming power in soft water, resilience in hard water, effectiveness at low temperatures, and inability to hydrolyze. Additionally, only small quantities of detergents made from LAS are required at a time. LAS is derived from petroleum and is cheaper than other surfactants.
LAB is a clear, colorless liquid with a distinct smell. It is made from C10-C13 or C11-C14 linear monoalkenes, which undergo sulfonation readily to form linear alkyl benzene sulfonates. Exposure to benzene through detergent use is associated with various health effects, including headaches, drowsiness, and dizziness. These effects occur following the inhalation of air containing more than 10 parts per million of benzene within a short time.
However, long-term exposure to benzene may elevate the risk of developing leukemia, attenuate the immune system, and cause anemia. Benzene also interferes with the reproductive system as noted in animal studies where the chemical was linked to infertility and fluctuations in the menstrual cycle. High levels of benzene in the body are excreted in the urine, thus causing the accumulation of the chemical in sewage systems. Additionally, the disposal of water from laundry processes involving LAB-based detergents leads to the accumulation of the compound in sewage works. The level of accumulation is dependent on the type of sewage treatment.
High concentrations of LAS at rates of 30,200 mg per kilogram of dry weight have been found in treated sludge. On the other hand, surface water has been reported to contain 416 μg/L, which exceeds the maximum allowable limit of 250 μg/L. These observations are attributed to the raw discharge of drainage into water bodies, which poses threats to aquatic life.
It has been reported that LAS harms the natural populations of soil fungi, bacteria, and actinomycetes. It was noted that LAS lowered the populations of soil bacteria such as Bacillus licheniformis. This effect was associated with the ability of LAS to destabilize bacterial proteases as well as lowering α-amylase action. These effects were evident even at amounts lower than the critical micellar concentration (CMC) of the LAS. The loss of enzymatic activity was linked to the electrostatic interactions characteristic of the anionic nature of LAS.
In fungi, a microarray analysis of Saccharomyces cerevisiae treated with LAS alongside SDS showed that the detergents interfered with the integrity of the cell membranes, altering carbon metabolism and triggering a rejoinder to oxidative stress. It has also been shown that the toxicity of LAS on algae is proportional to the hardness of the water and the number of carbon atoms in the alkyl chain.
Lakes and rivers, which are common sources of water for agricultural irrigation may also be contaminated with water containing LAS. Consequently, agricultural farms are polluted with the contaminant. Furthermore, using sewage sludge as fertilizer also introduces surfactant residues in terrestrial soils and agricultural commodities. LAS has been reported to inhibit the germination of rice seeds. The accumulation of LAS in water bodies has also been demonstrated to affect aquatic life by lowering sperm motility and inhibiting fertilization in fish.
Spot Removers Containing Carbon Tetrachloride
Carbon tetrachloride is a highly volatile clear liquid. Therefore, most carbon tetrachloride in the environment exists in gaseous form. The compound is not easily combustible. It has a sweet smell that is usually pronounced when the concentrations reach 10 parts per million. Carbon tetrachloride is a synthetic chemical hence does not come about inherently in the environment. It is used in the removal of spots from clothes, furniture, and carpets.
Other uses include as a cleaning liquid in the industry, as a degreasing agent in dry-cleaning businesses, grain fumigant, and fire extinguisher. The high vaporization rate of the compound implies that a significant proportion of the compound either during manufacture or use reaches the air as fumes. Carbon tetrachloride has been known to persist in the air for many years before it disintegrates into other chemicals. Therefore, it is a dangerous air pollutant. Small quantities of the compound can be found in surface and groundwater. However, a substantial proportion of carbon tetrachloride in surface water evaporates from the surface water into the air within a few weeks.
On the other hand, the compound may be entrapped in groundwater for extended durations. Carbon tetrachloride does not stick to soil particles. Therefore, it evaporates into the air following spillage on the ground. There is limited information regarding the accumulation of carbon tetrachloride in terrestrial and aquatic living organisms. However, it has other serious environmental concerns.
Carbon tetrachloride is categorized as a class I ozone-depleting compound. It is also a strong greenhouse gas. It is reported that carbon tetrachloride in the troposphere leads to global warming. The global warming potential of carbon tetrachloride is estimated to be 1400 times greater than that exhibited by carbon dioxide. The half-life of the compound is approximated at 34 years, hence it is regarded chemically inactive in the troposphere.
Nonetheless, carbon tetrachloride exhibits photochemical reactions in the stratosphere, which play a role in the diminution of the stratospheric ozone. For these reasons, carbon tetrachloride was identified as a hazardous compound that should be abolished from industrial processes during the Montreal Protocol in 1987. Therefore, carbon tetrachloride was banned in dispersive applications in developed nations in 1996 and 14 years later in developing nations as well. However, the Montreal Protocol exempted the use of the compound in non-dispersive applications. Despite the ban, it has been reported that carbon tetrafluoride levels are still high in the environment.
Soil Fumigants Containing Nitromethane
Nitromethane is a colorless, oleaginous substance with a strong, pungent smell. It is highly flammable and releases noxious fumes of nitrogen oxides following decomposition. It has a molecular weight of 61.4 and a melting point of -28.55°C. The density of nitromethane at 15°C 1.32 g/cm3, which is higher than the density of air. Nitromethane is used agriculturally as a soil fumigant to kill soil-borne pathogens and worms.
Other uses of the chemical included in the manufacture of industrial antimicrobials and pharmaceutical agents as well as fueling race car engines. As a soil fumigant, nitromethane exerts its toxicity by irreversible binding to the active site of the enzyme histidase thus inactivating it. In humans and animals, exposure to nitromethane causes skin irritation and disturbances of the central nervous system, thereby causing queasiness and dizziness. It is reported that nitromethane may be a human carcinogen with a lethal dose between 0.5 and 5 grams per kilogram of body weight.
Nitromethane is also associated with narcotic outcomes in humans and irritates the skin as well as the respiratory system. However, there are inadequate data to prove that nitromethane is a genotoxin. In water, nitromethane causes the death of fish and reduces the population of phytoplankton.
Benign Alternatives
To avoid the adverse outcomes associated with the above-mentioned chemical compounds, there is a need to consider the use of safer alternatives. Several solvents and groups of solvents that are considered safe include water, liquid polymers, ionic liquids, supercritical fluids, gas expanded liquids, and biomass-derived solvents. The classification of these substances as green solvents is based on the assumption that using them leads to enhanced environmental performance.
However, debates regarding the greenest solvent are still ongoing. The classification of green solvents does take into account the intended use of the solvent even though the effectiveness of the solvent in its anticipated use is vital. Before concluding that a solvent is green, it is important to consider various aspects concerning the intended use of the solvent. The first feature is the impact of the solvent on burning, for example, the heat of combustion, substances emitted, and their toxicity. It is critical to ascertain their solubility in water, ease of recycling, particularly the melting and boiling points as well as the number of other substances with the same boiling points.
Other factors that should be considered include drying capacity, formation of azeotropes, reactivity, ability to undergo biotreatment, impact on the aqueous environment, atmospheric impact, and workplace exposure risks. To ensure that the proposed solvent use matches the physical and chemical properties of the solvent, there is a need to merge the environmental assessment with approximations of the capacity of the solvent to further a reaction.
The use of LAB and LAS in detergents can be avoided by using fatty alcohols as surfactants. Fatty alcohols have a low toxicological profile and are considered safe. Another alternative to detergents is minimizing the use of soap by using alcohol-based surfactants in the personal care industry and promoting the sales of laundry liquids made of alcohol-based surfactants.
The use of tetrachloromethane in cleaning fluids and spot removers can be eliminated by using green solvents such as supercritical fluids and ionic liquids. Advances in high-pressure technologies entailing sub and supercritical fluids have made it possible to create novel products with unique traits as well as to design new processes that are environmentally friendly and sustainable. High pressure circumvents the legal restrictions of solvent residues in addition to the constraints of traditional solvents in chemical reactions.
Furthermore, supercritical fluids require less energy, which also improves their cost-efficiency. Supercritical fluids have already been applied in the manufacture of chemicals used in the food, pharmaceutical, and textile industries (dyeing and cleaning). It is possible to combine ionic solvents with supercritical fluids to produce solvents with exceptional qualities.
Alternatives to the use of nitromethane as a soil fumigant include using physical alternatives such as soil solarization, flooding, and steaming. These methods destroy soil pathogens, lower the viability of weed seeds, and reduce soil nematodes.
However, they are not always effective or workable. Consequently, anaerobic soil disinfestation (ASD) has been proposed to treat the soil. This method entails adding an organic carbon source to the soil, irrigating until the soil is saturated, and tarping with a layer that is impermeable to oxygen. The resultant conditions take advantage of inherent soil microbes that flourish in anaerobic environments and pile up toxins, which overwhelm an array of nematodes, weeds, fungi, and other microorganisms. Water, which is a green solvent, is the prevalent substance used in the process.
Conclusion
Merging technological advancement with the environmental safety is one of the major challenges of the millennium. Green chemistry seeks to enhance production efficiency while minimizing or getting rid of waste and emissions at their source as opposed to treating them after production. Numerous green solvents have been proposed for use in the manufacture of industrial, commercial, and domestic chemicals. However, before settling on a specific choice, it is necessary to consider their applicability in a specific situation. Important features to consider include the manufacturing efficiency when using the proposed alternative, the effects of exposure, and the environmental impact of the alternatives.
References
- Welton, T. Solvents and Sustainable Chemistry. Proc. R. Soc. A. 2015, 471(2183), 2015.
- Sheldon, RA. Green Chemistry and Resource Efficiency: Towards A Green Economy. Green Chem. 2016, 18, 3180-3183.
- Sheldon, R. A. The E Factor 25 Years On: The Rise of Green Chemistry and Sustainability. Green Chem. 2017, 19, 18-43.
- Syakti, A. D.; Asia, L.; Kanzari, F.; Umasangadji, H.; Lebarillier, S.; Oursel, B.; Garnier, C.; Malleret, L.; Ternois, Y.; Mille, G.; Doumenq, P. Indicators of Terrestrial Biogenic Hydrocarbon Contamination and Linear Alkyl Benzenes as Land-Base Pollution Tracers in Marine Sediments. Int. J. Environ. Sci. Technol. 2015, 12, 581-594.
- Jardak, K.; Drogui, P.; Daghrir, R. Surfactants in Aquatic and Terrestrial Environment: Occurrence, Behavior, and Treatment Processes. Environ. Sci. Pollut. Res. 2016, 23, 3195-3216.
- Zhou, J.; Wu, Z.; Yu, D.; Pang, Y.; Cai, H.; Liu, Y. Toxicity of Linear Alkylbenzene Sulfonate to Aquatic Plant Potamogeton perfoliatus L. Environ. Sci. Pollut. Res. 2018, 18, 1-9.
- Asok, A. K.; Fathima, P. A.; Jisha, M. S. Biodegradation of Linear Alkylbenzene Sulfonate (LAS) by Immobilized Pseudomonas sp. Adv. Chem. Eng. Sci. 2015, 5, 465-475.
- Oyoroko, E.; Ogamba, E. N. Effects of Detergent Containing Linear Alkyl Benzene Sulphonate on Behavioural Response of Heterobranchus bidorsalis, Clarias gariepinus and Heteroclarias. Biotechnol. Res. 2017, 3, 59-64.
- Jardine, A. J. Measuring Carbon Tetrachloride Atmospheric Mixing Ratios in the Central Amazon Rainforest. The Column, 2016, 12, 14-20.
- Hu, L.; Montzka, S. A.; Miller, B. R.; Andrews, A. E.; Miller, J. B.; Lehman, S. J.; Sweeney, C.; Miller, S. M.; Thoning, K.; Siso, C.; Atlas, E. L. Continued Emissions of Carbon Tetrachloride from the United States Nearly Two Decades After Its Phaseout For Dispersive Uses. Proc. Natl. Acad. Sci. 2016, 113, 2880-2285.
- Ou-Yang, C. F.; Chang, C. C.; Chen, S. P.; Chew, C.; Lee, B. R.; Chang, C. Y.; Montzka, S. A.; Dutton, G. S.; Butler, J. H.; Elkins, J. W.; Wang, J. L. Changes in the Levels and Variability of Halocarbons and the Compliance with the Montreal Protocol from an Urban View. Chemosphere, 2015, 138, 438-446.
- Yan, D.; Wang, Q.; Mao, L.; Ma, T.; Li, Y.; Ouyang, C.; Guo, M.; Cao, A. Interaction Between Nitrification, Denitrification and Nitrous Oxide Production in Fumigated Soils. Atmos. Environ. 2015, 103, 82-86.
- Sánchez, M.A.; Torres, G. C.; Mazzieri, V. A.; Pieck, C. L. Selective Hydrogenation of Fatty Acids and Methyl Esters of Fatty Acids to Obtain Fatty Alcohols–A Review. J. Chem. Technol. Biotechnol. 2017, 92, 27-42.
- Knez, Ž.; Markočič, E.; Leitgeb, M.; Primožič,; Hrnčič, M. K.; Škerget, M. Industrial Applications of Supercritical Fluids: A review. Energy. 2014, 77, 235-243.
- Marr, P. C.; Marr, A. C. Ionic Liquid Gel Materials: Applications in Green and Sustainable Chemistry. Green Chem. 2016, 18, 105-128.
- Strauss, S. L.; Kluepfel, D. A. Anaerobic Soil Disinfestation: A Chemical-Independent Approach to Pre-Plant Control of Plant Pathogens. J. Integr. Agric. 2015, 14, 2309-2318.