Introduction
The health problem considered in this paper is the epidemic of influenza, a small enveloped virus that is highly contagious. To date, the continuous spread of influenza viruses as well as the emergence of reassortant strains of animal origin pose a constant danger to people’s health. The researchers (1) state that clinical implications of influenza are associated with the involvement of lung tissue and pleura. It is possible to develop respiratory distress syndrome, in which patients need hospitalization and transfer to artificial lung ventilation within a day from the onset of symptoms. Some scholars (2) admit that the economic implications of an influenza pandemic require high annual costs, resulting in a decline in GDP. For some countries in South Asia and Africa, the decline in GDP as a result of the medical costs associated with influenza is equal to the level of the average economic growth rate of these states.
Treatments to be analyzed in this review are annual vaccinations that protect against three or four of the most common strains of the virus in a given area. The study will involve influenza vaccines of different compositions. These are four-component, three-component, and two-component vaccines that include an appropriate number of virus strains (3). The reason for performing this study is to evaluate the current evidence about the cost-effectiveness of influenza vaccination. The work aims to analyze data to find the economic effect of both medical expenses and indirect costs and comment on which vaccine is more cost-effective. It is possible to stipulate that the review follows a few objectives. Firstly, the aim is to find and synthesize the existing evidence to compare the cost-effectiveness of the trivalent versus quadrivalent influenza vaccine. Secondly, the review focuses on accumulating other relevant knowledge about the two vaccine types. The data focus on age group vaccination specifics, economic burden, reasons for limited use of influenza vaccines, safety and supply issues, health effects, and clinical outcome.
The various efforts performed by different countries in preparing and developing strategies to curb the influenza pandemic have been prioritized. According to Ortiz and Neuzil (4), influenza vaccines are hardly utilized in low and middle-income nations. The assessment of whether quadrivalent influenza vaccines are better than trivalent influenza vaccines has been done by evaluating the cost-effectiveness of the Quadrivalent Influenza vaccine (QIV) and Trivalent Influenza vaccine (TIV). There is a significant difference between the two types of influence vaccines. On the one hand, the scholars (5) admit that the TIV protects individuals from three influenza strains, including one B strain and two A strains. On the other hand, the name suggests that the QIV offers protection against two A and two B strains, making a total of four. That is why it seems reasonable to compare the effectiveness of these two approaches, and it is a task of this literature review.
The issue under analysis is not unique because many scholars focus on the topic. For example, the literature assessment (1) systematically evaluates the evidence of economic burden initiated on influenza in low and middle-income countries and shows financial consequences of influenza disease in the countries. It also examines why lower and middle-class countries underutilize influenza vaccines and the what vaccination costs they face. Still, it illustrates how the safety and availability of supply issues affect health results in regions that are more vulnerable to the disease. In addition, it provides different articles that cover numerous subjects on the disorders and can help conduct research. However, it is still reasonable to conduct a literature review to synthesize the existing knowledge on the cost-effectiveness of the TIV and QIV and conclude on which variant is better and why.
Methodology
The literature review methodology is used to undertake this study. The researchers (6) stipulate that this approach is suitable to locate and analyze international data on a specific issue to arrive at grounded conclusions. Thus, one can state that this methodology allows for summarizing and presenting an overview of the existing data. Consequently, it is reasonable to rely on the given approach to find and synthesize the data about the cost-effectiveness of the TIV and QIV.
The specific and detailed PICO question guides the whole research process. It is as follows: In patients from low- and middle-income countries (P), is quadrivalent influenza vaccine (I) or trivalent influence vaccine (C) is more cost-effective (O)? The proposed question should make it clear that the given literature review focuses on comparing TIV versus QIV. In other words, the primary task is to determine which strategy is more cost-effective.
The literature review dealt with the articles retrieved from Google Scholar. This resource was used to find additional credible and reliable studies. The rationale behind this statement is that Google Scholar provides access to many databases, including SciELO, NCBI, and others, and scholarly journals to locate relevant literature. The given work focuses on those articles that were written in English and between 2017-2021. Consequently, a convenience sampling technique was used to provide the paper with reports.
The searching strategy consisted of a few keywords, including trivalent influenza vaccine AND/OR quadrivalent influenza vaccine AND cost-effectiveness. As is evident, the Boolean operators AND were used to ensure that all the three key terms are present in the papers under analysis. Simultaneously, the OR operator denotes that any of the two key terms could be present in articles. The inclusion criteria were English language and the publication date between 2017-2021. There was an exception to this criterion because there were included three studies that were published before 2017, and they were considered because they offered valuable information. Simultaneously, a study was excluded from the literature review if reading its abstract revealed that it contained irrelevant content. Furthermore, a study was not included in the review if no full text was available. Finally, the articles were excluded if they only contained data about vaccination in high-income states. Thus, the literature review’s author is responsible for ensuring that appropriate studies are included in the analysis.
The interventions selected for the study consist in monitoring epidemiological data on the prevalence of the disease (disease burden) prior to vaccination. An intervention was also performed to determine the immunogenicity of the vaccines intended for use and to monitor the outcomes of vaccination. The comparator will be conducted on the basis of comparing the costs of vaccinations and treatment, payment of sick leave, and other manipulations with influenza patients. Monetary indicators of investments in vaccinations and treatment of patients will be compared.
Results
The selected search strategy resulted in the fact that 74 articles were initially obtained. The inclusion and exclusion criteria application resulted in the fact that 12 articles remained. The articles that were published earlier than 2017 were found by the references of the already selected papers. All the other studies were removed as per one of the stipulated exclusion criteria. In particular, the PRISMA chart below provides a detailed explanation of how the literature review has come to the specified number of articles. The chart is followed by the obtained results from individual themes that were mentioned in the Introduction section.
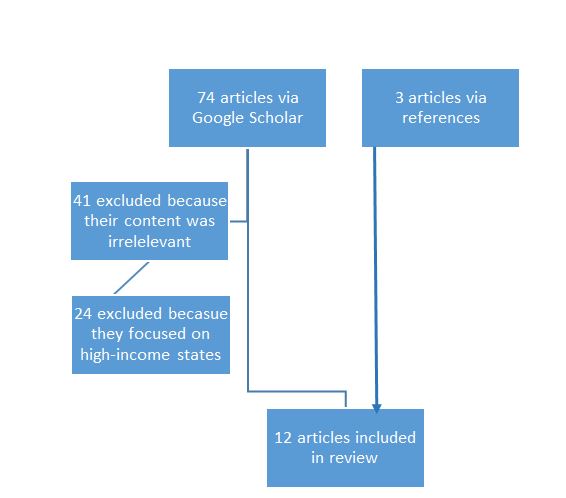
Table 1 lists all the articles that were included in the review, and their main findings.
Table 1: Literature Findings
Cost-effectiveness. In the beginning, it is worth admitting that QIV is a promising intervention to reduce an economic burden on healthcare systems. According to Lee, Bartsch, and Willig (7), this phenomenon contributes to better protection and cost savings at the same time. In particular, the researchers admit that relying on QIV instead of TIV can imply $3.1 billion in cost savings for society and approximately $290 million for third-party payers. Lee, Bartsch, and Willig (7) stipulate that this state of affairs is present irrespective of the fact that the cost of a single QIV dose is significantly higher than that of a TIV one. This information contributes to the fact that more and more countries and healthcare establishments consider implementing QIV.
In addition to that, other articles provide more specific results of comparing the cost-effectiveness of TIV and QIV. For example, the researchers (8) consider how these two vaccines are implemented in Brazil. These scholars (8) compared the cost-effectiveness of two vaccines under different conditions and identified that QIV could contribute to the savings of R$19,257 to R$22,768 using a lifetime horizon. The given study utilized a comprehensive and appropriate methodology and identified that QIV could contribute to better economic outcomes for patients.
The cost-effectiveness of QIV fluctuates among different countries because of differences in the study of disease transmission, co-morbidities, and unit costs. One can mention that the burden of influenza B decides the success of QIV or TIV among the targeted population and the country’s willingness to undergo the strains associated with the immunization (3). De Boer et al. (3), using an energetic modeling study authorized by the World Health Organization, recommends that QIV may be more financially savvy than TIV in the specified countries under certain conditions.
Quadrivalent Influenza vaccines provide a better health benefit, according to Hendriks et al. (12). If high attack rates were assumed, QIV would be cost-effective (3). Substituting QIV for TIV would mean an extra expense of 25–29% spending increment for occasional flu, resulting in an increase in the annual cost of immunization, leading to a rise in the National budget (13). The high costs associated with Quadrivalent Influenza vaccines might lead low and middle-income countries to select the Trivalent Influenza vaccines due to the low immunization costs. Low-income countries will prioritize the affordability of the vaccines, not their effectiveness (4). The QIV prices are significantly more than the prices of Trivalent Influenza vaccines (12). When the TIV doses are increased, the health gain is close to that associated with QIV; therefore, these low and middle-income countries choose to stick to the Trivalent Influenza vaccines as a better and cost-reducing option.
However, these countries need to decide if the health gain out-gauges the extra cost accrued from the utilization of QIV. Inoculating a bigger group of people with TIV has superior cash esteem regarding the effects on the wellbeing outcome. Still, QIV leads to a significant decrease in influenza prevalence, hospitalizations, and death (12). The production of seasonal Trivalent Influenza vaccines routinely uses a lot of time. Effectively finishing each vaccine production yearly, the vaccination process involves ideal and consistent correspondence between the WHO, manufacturers, and administrative units. Still, the introduction of QIV adds additional production risks and market delays (14). Newall et al. (14) discovered that immunization programs considered economically efficient are not implemented in developing countries. The cost-effective intervention is not always prioritized for financial backing due to insufficient financial resources (14). Decision-makers in these countries might prioritize other inoculation programs for the same budget increment, keep a level immunization financial plan, and opt for the cheap TIV instead of acquiring costly QIV influenza vaccinations.
Age group vaccination reviews. All age groups, children, adults, and the elderly, are infected by the influenza virus. However, the elderly are more susceptible to the risks associated with influenza disease; thus, influenza vaccination is highly advocated for them. However, the injection has been suggested for guardians and health workers since they often contact the infections. According to de Francisco (Shapovalova), Donadel, Jit, and Hutubessy (10), people of all ages with other medical conditions are in more danger of flu-related entanglements than the rest of the population. TIV is the recommended vaccine used in individuals with high risk. The use of high TIV doses has been proved to be effective and safe with no adverse effects. These vaccines, both the QIVs and TIVs, are considered safe for children and adults.
Children are susceptible to influenza infections; thus, a recommendation of influenza vaccination was implemented targeting an age group of 7 to 24 months. Neuzil et al. (13) states that for children above six months, TIVs are the recommended and authorized injections. However, QIV has been observed to contain the Yamagata and Victoria lineages of the B virus, which shows a more outstanding antibody defense against the immunogenicity and additional B strain than TVs (4). However, the health systems in low and middle-income countries lack proper structures for healthcare to administer and disseminate these vaccinations.
Economic burden. The scope of the burden of influenza varies with age and the health of the patient. For instance, in an investigation in South Africa, which is renowned for possessing the most extensive human immunodeficiency infection (HIV) pervasiveness, it was assessed that individuals who are HIV positive are more subjected to influenza-associated lower respiratory infections compared to HIV-uninfected people (12). Hendriks et al. (2018) suggest that HIV-infected people have a risk 4-8 of contracting the flu compared to HIV uninfected. The differences between financial constraints, socio-economic factors, health care financial plans, and the formulated budgetary plans do not allow the Cost-effectiveness outcomes to have the same effects between different countries (15). The complications related to influenza disease are higher in children and the elderly.
Those living in closed communities such as Refugee camps, small settlements, and plantations are more susceptible to these infections because proximity increases transmission rates. The economic factor is estimated by the assessment of the sickness funds (12). Seasonal Influenza immunization continues to pose a crisis in the U.S due to factors such as inadequate immunization vaccines (15). The cost-efficiency of Influenza vaccination varies between states due to the difference in influenza epidemiology, HIV prevalence, and unit cost (3). The author suggests that to assess whether Quadrivalent Influenza Vaccines are better and more cost-efficient than Trivalent Influenza vaccines depends on the budgetary impact, countries’ willingness to pay the threshold and influenza’s burden on the said country.
People with specific underlying health conditions comprise the elderly and young, who are considered to be at higher risk of developing multifaceted complications. In addition, influenza poses a critical financial weight that is accrued from medical productivity losses and costs. In low-and middle-income nations, expenditures stemming from influenza may have a huge financial impact that has been surveyed at 3–7% of (GDP) per capita, diverged from simply 0.05–0.14% of GDP per capita in revenue generation of the nations (12). The previously done economic analysis on influenza immunization had focused on high-income countries overlooking the monetary effects of influenza vaccination in Low and middle-revenue nations. According to the researchers (12), the economic burden of the infection covers the instant expenses to the health services, people and the unintended budgets felt in the output losses, which affect the broader economy. The scientists claim (12) that the direct costs in middle and low-income countries Compared to high-income economies are lower and productivity losses higher. Thus, due to different socio-economic factors, co-morbidities, budget impacts, infrastructure, and health care plans, the economic cost value of the Trivalent influenza vaccines and Quadrivalent influenza vaccines vary.
Reasons for limited vaccine use. WHO’s routine immunization services do not reach the Low and middle-income countries due to poor infrastructure to reach the people in these communities and administer the immunizations. Ortiz and Neuzil (4) mention that the systems used to evaluate, assess, regulate and administer these vaccines are weak thus cannot support the set immunization program for adults and children. Neuzil et al. (13) say that the lack of clarity on the importance of prioritizing the immunization, insufficient evidence on the economic burden it poses, and the poor data on the vaccine’s efficacy has led to its underused in these low and middle-income states. Gupta et al. (11) state that to increase the efficiency of the vaccine being administered, it is vital to understand the epidemiology of this disease is crucial. According to Ortiz & Neuzil (4), effective surveillance needs to be put in place to establish the disease burden and overall risk in the targeted groups such as children, older people, and pregnant women.
The already available national data showing the stain, disease burden, and timing can effectively help reduce the constraints that have made Some Low and middle-income countries prefer to administer the periodic Influenza vaccine in their vaccination package. They are also considering whether this program should involve Trivalent Influenza vaccines or Quadrivalent Influenza vaccines (9). However, identifying target populations, patients with chronic conditions, and the effective vaccination strategies that will target specific endangered clusters of individuals in the low and Middle-Income countries can be challenging. According to de Boer et al. (9), for the Low and middle-income countries to consider the practice of the influenza vaccines, data on health outcomes, such as severe illness and mortality, are needed. The lack of a sound vaccination dissemination system that regularly monitors the infections, the poor infrastructure, and the financial constraints also cause the limited use of influenza vaccines.
Safety and supply issues. The routine seasonal production of Trivalent Influenza vaccines takes a considerable amount of time. To effectively finish each progression in the flu immunization producing cycle, timely and regular correspondence between the WHO, manufacturer, and administrative authority has required the introduction of Quadrivalent Influenza vaccines would mean additional market delays and production risks (14). Vaccine creation begins one year before the set date of the agreement in the Northern and Southern hemispheres (12). The vaccine production steps are time-critical and require an exceptional manufacturing environment. Thus, replacing the trivalent Influenza vaccines in the market comes with production risks and untimely market delays. This change could pose a lot of practical difficulties in the market. Safety policies of influenza vaccines need to be well understood to reduce the adverse effect of a reaction (15). The vaccine’s safety is highly critical in creating public confidence in the vaccination programs. The flu shots are advocated for children, adults with other medical issues, and older persons who are highly susceptible to infections. These vaccines are required to meet the required health standards and guidelines since they are provided to healthy individuals such as children.
The WHO monitors these vaccines before they are issued by different states. The management on the economic assessment of influenza vaccination, set up by WHO, defines heretical concepts and the best methodological practices that facilitate efficient guidance on influenza vaccination evaluation in Low and middle-income nations (15). This guideline includes an economic assessment of the Influenza vaccine in terms of the estimates of adverse events that may occur after immunization (12). In low-income areas, trivalent influenza vaccines are better since they are affordable, practical, and logistically feasible (12). During clinical trials, the recognition of potentially severe adverse reactions should be considered. These potential risks can cause drastic deviations in the invention course, specifically if the responses are deadly and put lives at risk (15). The safety of these vaccines is crucial because it’s administered to many healthy persons; thus, the WHO should ensure the manufactured vaccines meet the required standards. The decision to replace the Trivalent Influenza vaccines with QIV depends on the transition on the country’s burden and the resources available for the transitions in the manufacturing process.
Health effects and clinical outcome. Influenza causes premature death, and individuals who are HIV positive have a higher mortality rate if infected by influenza than the Non-HIV infected individuals (1). Vaccination reduces clinical visits, hospitalizations, and mortality rates. According to the research done by Neuzil et al. (13) and de Boer et al. (9), the advantage of TIV over QIV was assessed to be a 13.1% decrease of suggestive flu and an 18.0% decrease of flu-associated death. Introducing Quadrivalent Influenza vaccines would reduce influenza cases, which will lead to fewer deaths. In Agincourt SA, when the QIV vaccine was used over TIV, the outcomes gave an estimation of a 13.1% decrease of suggestive flu and an 18.0% decrease of flu-related mortality (1). From the data collected in the two influenza seasons by Gupta et al. (11), those vaccinated with QIV, 21364 of 53627 (5.3%) and 6365 of 133147 (6.5%) were hospitalized distinguished to 7873 of 111297 (8.4%) and 4423 of 43222 (8.3%) of those immunized with TIV S2 and S1, separately.
Seasons with moderately high flu B action, Quadrivalent Influenza Vaccine results appeared to be more compelling than Trivalent Influenza vaccines (11). In South Africa, for example, the cost-efficiency of TIV was as shown from the studies by de Boer et al. (2018), is estimated depending on the assumed attack rate. A review conducted in Australia, Vietnam, and South Africa, by de Boer et al. (3), introducing 6% of TIV vaccination while assuming a SAR of 5%, showed the decrease in the predominance of the seasonal flu locally by 48.4–50.2%. These reductions included hospitalizations, death, and clinical visits and were the same across all age groups. Additional doses of TIV administered to large groups share a close to the higher outcome linked with QIVs.
Vaccination cost. The cost incurred can determine the charges of the Influenza vaccine depending on the form of vaccination being directed. The Quadrivalent inactivated vaccine [QIV]) and trivalent inactivated vaccines (TIV) differ in prices, and most low and middle-income countries prefer the Trivalent Influenza vaccines due to their low costs (13). The direct service delivery cost associated with the cost of the cost related to doctor’s expenses and visits, vaccination of the vaccines in health care faculties can also lead to an increment in the vaccine cost when related to the vaccines administered in non-medical administrations such as pharmacies. However, inoculation can be done and managed by lower-wage staff like qualified medical caretakers to decrease medical expenses.
Proof on the expense adequacy of QIV shows that supplanting TIV with QIV would be a significant move economically and beneficial in public health. However, low and middle-class countries have low resources, and their budget can hardly support the transition (12). De Francisco (Shapovalova), Donadel, Jit, and Hutubessy (10) add that the potential additional benefits derived from Quadrivalent Influenza vaccines are therefore not recognized in these countries because they cannot afford the costs.
Yearly, the expense associated with vaccination, including direct clinical consideration and lost profit, has been assessed at $26.7 billion (12). The cost of TIV is lower compared to QIV, thus is preferred in numerous Low and middle-income nations. From the research reports made by de Boer et al. (3), it’s observed that the practice of an extra quantity of TIV prescriptions in low and middle-class countries results in a very close to or higher number of avoided cases of influenza infections than what is achieved through QIV. To determine which vaccine is more effective at a lower cost affordable, some factors such as illness and the practical realities of product availability to be used for immunization systems are considered. Influenza-related costs are grouped into healthcare and non-medical care costs.
The medical services cost constitutes GDP costs, medication expenses, and others. In contrast, non-medical care expenses incorporate expenses incurred from time and profitability losses resulting from work loss (1). The Quadrivalent Influenza vaccine prices are higher than Trivalent influenza vaccines from the study reports across the countries. From the indication on the affordability of QIV, deciding on the selection of this vaccine would be a significant choice, especially in enhancing public health. However, in low and middle-income countries with a fixed budget and inadequate resources, this move would cause many financial constraints; that’s why most of these countries consider TIV to be the most attractive option.
Discussion
Each of the articles contained data confirming the economic advantages of these vaccination types. The points extracted from the search were related to the cost of cases, the number of cases, and the intensity of symptoms. The results showed that the findings of specially planned studies to assess the economic effectiveness of influenza vaccination demonstrated the profitability of vaccination prevention. At the same time, it was shown that the economic effect of vaccination increases in proportion to the increase in the incidence of influenza.
Sufficient evidence has proved that the application of QIV instead of TIV can lead to significant economic benefits for entire societies and healthcare systems. It is worth admitting that the literature review deals with figures in different currencies, including American dollars and Brazilian real. There is no necessity to convert any of these currencies to find a common denominator. Since the focus of the literature review is to deal with the cost-effectiveness of two vaccination options, it can only be sufficient to compare the figures. Since many articles reveal that QIV is associated with lower expenses when compared to TIV costs, it is possible to confirm the cost-effectiveness of this intervention.
At the same time, the initial hypothesis was confirmed that the influenza epidemic has the most significant economic consequences for developing countries. In comparison with the studies conducted by European scientists, the results obtained by African and Asian researchers differ. Despite the reduced prevalence of vaccination, in these countries, it has the greatest economic efficiency due to the low GDP of the state.
Clinical trials to measure vaccine efficacies are being conducted, and efforts to develop an influenza vaccine that is more effective and durable in preventing Influenza disease are underway. In low and middle-income societies, vaccination programs and health care systems are weak. The vaccine deliveries are affected by the poor infrastructure. High-income countries can afford to substitute seasonal trivalent influenza vaccines with quadrivalent vaccines due to resource availability. The Quadrivalent vaccines have an additional health benefit; they are preferred more in the countries that can afford their costs (4). However, in countries of lower income, the National budgetary plans and considerations lead to the preference of TIVs due to their low cost. Therefore, determining the most effective vaccine requires the efforts of countries to have knowledge on the benefits and disadvantages of both TIVs and QIVs (10). Influenza A strain causes severe influenza morbidity and mortality rates, according to Newall et al. (14). Thus, QIVs would be the most financially effective vaccine but, the overall budgetary impact of QIV is higher than that of TIVs.
Due to the lack of funding and financial constraints, high cost-effective vaccines are not prioritized in these countries. When considering suitable vaccines to use in low and middle-income regions, individuals mandated to make decisions feel the context-specific limitations, such as human limitations and available health facilities. The indirect patient efficiency and direct transportation costs incurred during the vaccine dissemination should be considered while assessing the expense of an inoculation procedure and strategy (13). The Inclusion of productivity costs, dictated by lost time opportunity to patients or guardians, add to the expenses inferable to the program, which will help countries in their cost-effectiveness estimation.
Counting transportation costs paid by people to go for inoculation in clinics will likewise add to the community expenses of the flu immunization strategies (4). However, the individual cost in most cases is insignificant thus does not need to be included in the cost-effectiveness estimation strategies. TIV and QIV’s economic value depends on many factors such as influenza epidemiology, vaccine price, and budgetary impact. Assessing these factors will help a country decide on the best vaccination program beneficial both health-wise and helps save the cost.
The strengths of my review are a wide selection of geographical areas used in the study. The economic effects of vaccination were considered not only in Europe but also in developing countries. The weaknesses of the study are the use of data only for large states. This is due to the insufficient number of observations conducted on the economic effects of vaccination in small Asian and African countries.
Policy implications of this study substantiate the costs associated with immunization of people of working age. The data obtained also take into account the specifics of economic processes and the level of medical development of the countries represented in the study. Reasons for further research are to keep investigating the issue not only using an empirical method but also using situation modeling. In addition, further studies can be carried out using the methodology for calculating the economic profitability of vaccination at different levels of seasonal influenza incidence.
Conclusion
The given paper has conducted a literature review and compared the cost-effectiveness of trivalent and quadrivalent influenza vaccination options. The appropriate methodology allowed for collecting and synthesizing the relevant evidence. In turn, this fact contributed to finding the evidence to comment on the leading research objective and describe a few related topics. According to the collected and synthesized data, quadrivalent influenza vaccination is more cost-effective because it implies financial savings. Researchers from different middle-income countries demonstrated that the intervention under consideration implied savings for societies and healthcare systems, in general, and individuals, in particular. These findings indicate that various countries should consider implementing quadrivalent influenza vaccines irrespective of the fact that a single dose of this medicine is higher than that of a trivalent one. The difference arises because quadrivalent vaccines are more efficient, which simultaneously implies economic benefits.
References
Arefin, M, Masaki, T, Kabir, K, Tanimoto, J. Interplay between cost and effectiveness in influenza vaccine uptake: a vaccination game approach. Proc. R. Soc. A: Math. Phys. Eng. Sci. 2019;475(2232):20190608.
Dabestani, NM, Leidner, AJ, Seiber, EE, Kim, H, Graitcer, SB, Foppa, IM, et al. A review of the cost-effectiveness of adult influenza vaccination and other preventive services. Prev. Med. 2019;126:105734.
de Boer, P, Kelso, J, Halder, N, Nguyen, T, Moyes, J, Cohen, C, et al. The cost-effectiveness of trivalent and quadrivalent influenza vaccination in communities in South Africa, Vietnam, and Australia. Vaccine 2018;36(7):997-1007.
Ortiz, JR, Neuzil KM. Influenza immunization in low- and middle-income countries: preparing for next-generation influenza vaccines. J. Infect. Dis. 2019;219(Supplement 1):S97-S106.
Coleman, BL, Snaderson, R, Haag, MDM, McGovern, I. Effectiveness of the MF59-adjuvanted trivalent or quadrivalent seasonal influenza vaccine among adults 65 years of age or older, a systematic review and meta-analysis. Influenza Other Respir Viruses 2021;15(6):813-823.
Munn, Z, Peters, MDJ, Stern, C, Tufanaru, C, McArthur, A, Aromataris, E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med. Res. Methodol. 2018;18(143):1-7.
Lee, BY, Bartsch, SM, Willig, AM. The economic value of a quadrivalent versus trivalent influenza vaccine. Vaccine 2012;30(52):7443-7446.
Van Bellinghen, LA, Marijam, A, de Araujo, GTB, Gomez, J, Van Vlaenderen, I. Cost-utility of quadrivalent versus trivalent influenza vaccine in Brazil – comparison of outcomes from different static model types. Braz J Infect Dis 2018;22(1):1-10.
de Boer, P, van Maanen, B, Damm, O, Ultsch, B, Dolk, F, Crépey, P, et al. A systematic review of the health economic consequences of quadrivalent influenza vaccination. Expert Rev. Pharmacoeconomics Outcomes Res. 2017;17(3);249-265.
de Francisco (Shapovalova), N, Donadel, M, Jit, M, Hutubessy, R. A systematic review of the social and economic burden of influenza in low- and middle-income countries. Vaccine 2015;33(48):6537-6544.
Gupta, V, Dawood, F, Muangchana, C, Lan, P, Xeuatvongsa, A, Sovann, L, et al. Influenza vaccination guidelines and vaccine sales in southeast Asia: 2008–2011. PLoS ONE 2012;7(12):e52842.
Hendriks, J, Hutubessy, R, Grohmann, G, Torelli, G, Friede, M, Kieny, M. Quadrivalent influenza vaccines in low and middle income countries: cost-effectiveness, affordability and availability. Vaccine 2018;36(28):3993-3997.
Neuzil, K, Bresee, J, de la Hoz, F, Johansen, K, Karron, R, Krishnan, A, et al. Data and product needs for influenza immunization programs in low- and middle-income countries: rationale and main conclusions of the WHO preferred product characteristics for next-generation influenza vaccines. Vaccine 2017;35(43):5734-5737.
Newall, A, Chaiyakunapruk, N, Lambach, P, Hutubessy, R. WHO guide on the economic evaluation of influenza vaccination. Influenza Other Respir Virusess 2017;12(2):211-219.
Wiley, S. Seasonal influenza vaccine guidelines, 2018-2019.Nursing 2018;48(11):11-13.