Introduction
Indigotine is an organic compound that is a reddish-blue food additive. Naturally, the compound is derived from sea snails and plants belonging to the genus Indigofera (Boer 2014). Production from organisms is not cost-effective because thousands of sea snails or plants are required to produce a pound of indigotine. Hence, the synthetic process of producing indigotine though benzene ammonification and aniline sulfonation is cost-effective (Preparation of indigo 2018). Thus, this assessment of the indigotine focuses on chemical structure, synthesis, applications, regulations, toxicology, the theoretical maximum daily intake, the estimated daily intake, and risk analysis.
Name and Structure
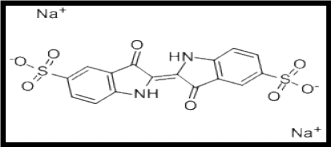
The chemical (IUPAC) name of indigotine is 3,3′-dioxo-2,2′-bisindolyden-5,5′-disulfonic acid disodium salt. Other synonyms of indigotine are brilliant indigo, indigo carmine, FD&C-blue-2, CI food blue 1, and sicovit indigotin 85 (Food and Agricultural Organization 2010). Indigotine has a chemical formula of C16H8N2Na2O8S2, a molecular mass of 466.36 g/mole, and a molecular structure is shown in Figure 1 below. Physical properties show that the indigotine is a blue or purple powder that is soluble in water with a melting point of 300°C. It is classified as a food colorant and assigned a code number 132 in the range of blue and violet colors (Federal Register of Legislation 2018).
Manufacture and Specifications
Raw materials required for the synthesis of indigotine are benzene, formaldehyde, ammonia, sodium hydroxide, sodamide, potassium hydroxide, and sulfuric acid (Preparation of indigo 2018). The synthesis of indigotine occurs in five stages, which entail the formation of phenylglycine, the fusion of molten mixture, the oxidation of indigo, the acidification of indigo, and the purification of indigotine. The formation of phenylglycine is the first stage involves the combination of benzene, ammonia, and formaldehyde. In the second stage, which is the fusion stage, sodamide, phenylglycine, sodium hydroxide, and potassium hydroxide reacts in high temperatures of 500°C to form indoxylate salts (Preparation of indigo 2018). In the third stage of oxidation, indoxylate salts convert into indigo under alkaline conditions, 70°C, and ammonia pressure. Subsequently, acidification with 30% sulfuric acid to form indigotine occurs in the fourth stage. To remove contaminants, which are mainly alkali salts, ammonium salts, and sulfuric acid, indigotine is boiled with pyridine and hydrochloric acid. Table 1 below highlights the physical and chemical properties of the indigotine.
Table 1: Chemical and Physical Properties of Indigotine. (Indigo carmine 2016, para. 1).
Food Use and Applications
Indigotine is a food additive that has been approved in the United States, Europe, and Australia. The joint committee of the World Health Organization and the Food and Agricultural Organization has established an acceptable daily level of indigotine intake as a food additive. The common foods colored with indigotine are cereals, candies, and cakes (Saltmarsh 2013). Since indigotine is soluble in water, it is an appropriate food colorant, which provides a uniform coloring in diverse foods. In addition to coloring food, indigotine has numerous uses. In medicine, indigotine is an analytic agent used in the diagnosis of renal function by detecting orifices through cystoscopy and catheterization (Saltmarsh 2013). Moreover, it detects leakage of amniotic fluid in pregnant women. In the laboratory, indigotine is used as a pH indicator for it turns yellow at a pH of 13 and blue at a pH of 11.4. Although heat does not influence the color of indigotine, oxygen and light decolorize it, whereas pH influences its color.
Regulations/Food Standard Information
Food standards of Australia recognize food additives and provide regulations and standards stipulating their use in the food industry. Specifically, indigotine falls under the standard 1.3.1 of food additives in the Australia New Zealand Food Standards Code. The standard has come into effect since March 1, 2016, when it was published in the Food Standards Australia New Zealand Act (1991) (Federal Register of Legislation 2017). Schedule 15 provides regulations that permit and regulates the use of indigotine as a food colorant. Clause 4 and item number 3 list the maximum permitted levels of indigotine in beverage and food, as shown in Table 2.
Table 2: Comparison of Permitted and Theoretical Maximum Level of Indigotine.
Toxicological Assessment and Adi
Several toxicological studies done on bacteria, rats, rabbits, and humans confirm that indigotine is a safe food additive as assessed by the Joint Expert Committee on Food Additives. In a mutagenic study where a concentration of indigotine (0.5g/100ml) was added to a culture of Escherichia coli, the outcome did not establish a mutagenic effect (International Programme on Chemical Safety 2018). Since the indigotine undergoes metabolisms in the body, it generates Isatin-5-sulfonic acid as a major metabolite. An in vitro study was done to determine toxicity by feeding rats on 0.25%, 0.5%, 1%, and 2% of the metabolite for 13 weeks. Histopathological examinations of vital organs revealed that the metabolite has no toxicological effects on rats.
Teratogenic studies on rats and rabbits did not show a significant effect of indigotine on the growth and development of offspring. In another study, mice were fed on 25mg, 75mg, and 250 mg/kg of indigotine daily during the 6th-18th days of gestation and eventually sacrificed on the 29th day of gestation. Behavioral, physical, histopathological, and teratogenic findings did not show the harmful effects of indigotine (International Programme on Chemical Safety 2018). A similar study was replicated on pregnant rabbits, which were fed on 25mg, 75mg, and 250mg of indigotine from the 6th to 18th days of gestation. The findings also did not exhibit negative effects on behavior, weight-gain, fetal development, and maternal health.
Acute toxicity studies established that mice have a lethal oral dose (LD50) of 2500 mg/kg and a lethal subcutaneous dose of 405 mg/kg. Comparatively, rats have a lethal oral dose of 2000 mg/kg and a lethal intravenous dose of 93 mg/kg. Additionally, 250 mg/kg of indigotine administered subcutaneously to rats twice a day for three days caused them to die on the fourth day (International Programme on Chemical Safety 2018). A study done on pigs by feeding them on 150, 450 and 1350 mg/kg of indigotine in diet revealed that high doses of 1350mg/kg caused hemoglobin levels to reduce and the dose of 150 mg/kg caused liver abscesses.
Since the observed effects on animals extrapolate to human effects, they formed the basis for the approval of the indigotine as a safe food colorant (Rovina, Siddiquee & Shaarani 2016). The acceptable daily intake (ADI) was established in a long-term study on rats where 500 mg/kg was found to be without adverse effects on growth and was divided by an uncertainty factor of 100 (European Food Safety Authority 2014). Thus, the ADI for indigotine was determined to be 5 mg/kg of body weight (500 mg/kg/100).
Tmdi Calculation
To calculate the theoretical maximum dietary intake (TMDI), the required data for the mean grams of food intake were derived from the statistics regarding Australian dietary guidelines (Australian Bureau of Statistics 2016). The values of the maximum allowance level were obtained from the Federal Register of Legislation (2017), which indicates that beverage and food should have a maximum allowance of 70 mg/kg and 290 mg/kg respectively. Dietary intake of indigotine was calculated for each food group by multiplying kilograms of food or beverage by respective maximum allowance level (Food and Agricultural Organization 2014). Table 3 below illustrates the calculations of the dietary intake of indigotine for each food group and the TMDI.
Table 3: Calculations of TMDI.
Edi Calculation
The estimated dietary intake (EDI) was established by taking a typical diet of a person who has a high-risk of consuming higher levels of indigotine than an ordinary person consumes. The amount of food in kilograms was determined and multiplied by the stipulated maximum permitted level. The total amount of dietary intake of indigotine was divided by the total amount of food in kilograms to obtain the EDI in mg/kg, as depicted in the following Table 4.
Table 4: Calculations of the EDI.
Risk Analysis and Conclusion
The analysis of indigotine shows that it has the TMDI of 420 mg/kg, the EDI of 260.03 mg/kg, and the ADI of 5 mg/kg of body weight. For an average bodyweight of 60kg, ADI for a person is 300 mg. According to Pressman et al. (2017), the level of a food additive is safe when the EDI is lower than the ADI, but unsafe when the EDI is greater than the ADI. In this case, calculations of indigotine demonstrate that its level in food is safe because the EDI (260 mg/kg) is less than the ADI (300mg/kg). Since the TMDI is greater than the ADI, it implies that there is the possibility of the EDI to exceed the ADI and make the consumption of indigotine unsafe in the food industry.
These outcomes of the TMDI, the ADI, and the EDI are based on the assumption that a person is 60kg and adhere to dietary guidelines of Australia. The high-risk foods are cereals, dairy products, candies, and beverages. Additionally, the high-risk groups are individuals aged between 19 and 50 years for they are active consumers of high-risk foods. Based on toxicological studies, the likely implications are that indigotine would have negative effects on consumers for it has the potential of reducing hemoglobin, causing a liver abscess, and triggering cancer. Hence, to avert the negative effects of indigotine, the assessment recommends a reduction of the ADI to 2.5mg/kg to lower the risk of exceeding the EDI in high-risk individuals. Conclusively, the indigotine is a safe food colorant, which requires prudent use to prevent potential negative effects.
References
Australian Bureau of Statistics 2016, Australian health survey: consumption of food groups from the Australian dietary guidelines, 2011-12. Web.
Boer, L 2014, ‘Biotechnological production of colourants’, Advances in Biochemical Engineering/Biotechnology, vol. 14, no. 1, pp. 51-89.
European Food Safety Authority 2014, Scientific opinion on the re-evaluation of indigo carmine (E 132) as a food additive. Web.
Federal Register of Legislation 2017, Australia New Zealand Food Standards Code: standard 1.3.1-food additives. Web.
Food and Agricultural Organization 2010, Indigotine. Web.
Food and Agricultural Organization 2014, Guidelines for the simple evaluation of dietary exposure to food additives. Web.
Indigo carmine2018. Web.
International Programme on Chemical Safety 2018, Indigotine, Web.
Preparation of indigo 2018, Web.
Pressman, P, Clemens, R, Hayes, W & Reddy, C 2017, ‘Food additive safety: a review of toxicologic and regulatory issues’, Toxicology Research and Application, vol. 1, no. 1, pp. 1-22.
Rovina, K, Siddiquee, S & Shaarani, S 2016, ‘Extraction, analytical and advanced methods for detection of Allura red AC (E129) in food and beverages products’, Frontiers in Microbiology, vol. 7, no. 798, pp. 1-13.
Saltmarsh, M (ed.) 2013, Essential guide to food additives, 4th edn, Royal Society of Chemistry, London.