Introduction
A bacteriophage is a virus that can infect and replicate within a bacteria. Bacteriophages are made up of up of proteins that surround a DNA or RNA genetic material. They replicate within the bacterium cells after the injection of their genome into the bacterium’s cytoplasm. They can only replicate within the bacterium cells and are recognized as one of the most abundant biological agents on the earth. The bacteriophages are species-specific and only infect a specific strain of bacteria species. Once a bacteriophage gets attached to the host, it undergoes a lytic or lysogenic cycle. These are the two replication cycles that bacteriophages undergo when attached to the host. They bind to the host’s genetic material leading to either the incorporation into the host cell or destruction of the cell.
In a lytic replication cycle, the bacteriophage introduces its genetic material into the cytoplasm of the host. In this cycle, the bacteriophage kills the host and lyses the bacterial cell wall. This lysing causes zones of clearing in the agar plate with the bacterial cells. The lysogenic cycle causes the insertion of its genetic material into the bacterial cell’s DNA. The bacteriophage and bacterium thus replicate together, and therefore no lysis is evidenced. Thus, the lysogenic pathway can be applied in genetic engineering because it can insert genes into the host’s DNA material. Techniques to identify and distinguish lysogenic from lytic strains are therefore fundamental in the field of medicine. In this experiment, two strains of Staphylococcus epidermidis (A108 and A109) were used. The bacteria species is part of normal human flora. The strains used were either able to undergo a lytic or lysogenic pathway. It is a gram-negative bacteria and one of the many species belonging to the Staphylococcus genus.
A temperate bacteriophage can undergo both the lytic and lysogenic processes. The temperate phage’s infection can lead to the bacterium’s death or incorporate the temperate phage genome into the host’s cell. When the temperate phages enter the lytic cycle, they can serve an advantage of killing infectious bacteria within the cells of human beings. It can thus help in neutralizing contagious agents. The temperate phages in the lysogenic cycle can be used in recombinant DNA technology to design essential genes that can be used to create crucial biomolecules like insulin. The lysogenic bacteriophage can thus be used to insert vital genes in the bacteria, and the genes can be replicated on a grander scale. The disadvantage of temperate phages is that through the lysogenic cycle, they can insert into the genes of the bacteria, making them mutant. The mutant bacteria may be resistant to antibiotics. It may make it hard for the pharmacological design of drugs due to these mutations brought about by lysogeny. In the experiment, the titer of the sample was counted to give an estimate of the titer.
Aim
This study targeted to determine which A108 and A109 bacterial lawn cultures contained the lysogenic strain of Staphylococcus epidermidis. The experiment also aimed to experiment with bacteriophage titer using the pour plate method to prepare nutrient layer agar. The experiment aimed to distinguish between lysogenic and lytic strains of Staphylococcus epidermidis.
Results
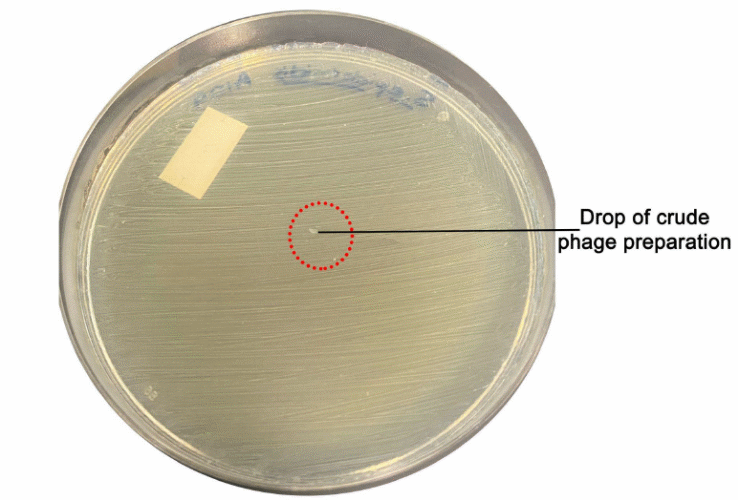
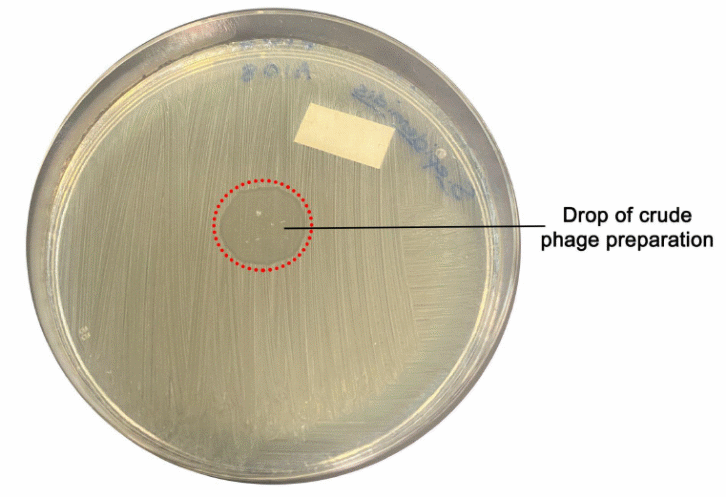
A drop of A109 was added to the A108 field. It was observed that there was a clearing surrounding the A109 drop. A drop of A108 was added to the A109 field. There was no observed clearing surrounding the A108 drop. It confirms that A109 is a lysogenic strain and causes clearing. The experimental pictures help in differentiating the lysogenic and lytic strains of Staphylococcus epidermidis.
The pour plate method was used to prepare a nutrient layer agar under different dilution factors. The lysis was counted, and their number was used to estimate the titers by use of the formulae; the count/volume of the mixture added×dilution factor.
Table 1: Titre Calculations Based on the Nutrient Layer Agar.
Discussion
In the agar plate, A109 is the lysogenic strain evidenced by a clearing shown around it. In the A109 strain of Staphylococcus epidermidis, the outer area of the drop has more access to the nutrients and has a decreased competition for a substrate. A109 dropped in the A108 area has the capability of infecting the A108 strain. The infected A108 strain thus undergoes the lytic cycle, and in the process, the surrounding A108 bacteria strain dies. The dying of the A108 thus causes a clearing seen around the A109 drop. The A109 undergoes the lysogenic process because of the increased nutrient competition. The A108 entered the lysogenic cycle rapidly replicated, infecting the surrounding A108, which in turn underwent the lytic phase.
No clearing is seen in the A108 plate. When the A108 strain is added to the A108 area, no clearing is observed. The A108 strain added to the A109 is not lysogenic, and there is no incorporation of the genetic material in order to produce a phage that can target the A109 bacteriophage. There is thus no clearing as the surrounding A109 bacteriophage cannot undergo a lytic pathway. The lysis of the bacteria is what causes the clearing. The clearing was seen around A109, meaning the bacteriophage was a lysogenic strain. There is no clearing seen around the A108 droplet, indicating that the strain is not lysogenic.
The titer in the sample was calculated by the count/volume of the mixture added×dilution factor. Only counts between 30 and 300 are important in estimating the titer used in the model. The growth area is too concentrated, and thus the strains used face a tight competition for nutrients. The close concentration makes it hard to estimate the number of titters in the sample correctly. The estimation of the titer provides a clue to the titer in the experiment. The person performing the experiment should strive to make correct counts so that the reliability of the results can be improved.
When a bacteriophage infects a bacteria, it will undergo either a lytic or lysogenic cycle. In the lytic cycle, the bacteriophage breaks down the genetic material of the host bacteria. The bacterium thus bursts and dies and releasing the new bacteriophage. In the lysogenic process, the genomic material of the bacteriophage gets incorporated into the DNA of the bacterium. The bacterium replicates along with the bacteriophage, and no lysis is evidenced. The incorporation of the DNA into the host material is termed lysogeny. A temperate bacteriophage undergoes both the lytic and lysogenic pathways. The Staphylococcus epidermidis can undergo both cycles indicated by the experimental results. A temperate bacteriophage undergoes a lytic cycle under the appropriate conditions and a lysogenic process when there is increased competition for nutrients. More experiments should be done on temperate phages to understand better their physiological behavior. The bacteriophages are important biological agents that can be used in research and help synthesize important artificial human biomolecules.
Conclusion
The purpose of the experiment was to investigate which bacterial lawn culture contained lysogenic bacteria. The agar plate method can be effectively used to distinguish which strains of bacteriophage are lysogenic and lytic. It was observed that the A109 sample contained the lysogenic bacteria, while the A108 contained the lytic bacteria. It can also be used in determining phage tittering. In this experiment, a titer of about 4.8×104 phages was approximated. One of the limitations is that the counts may be too concentrated or many for different volumes of samples used. Thus, inaccurate and unreliable results may be obtained during titer calculation. The ability to distinguish between lysogenic and lytic bacteria can be applied in recombinant genetic technology to identify lysogenic bacterias crucial in creating important human biomolecules like insulin. More research needs to be done to better understand the genetic recombination between the bacteriophages and the host. These findings help explain how the agar plate method can distinguish the lytic and lysogenic samples. The conclusions can be applied in microbiology and biochemistry to understand better the bacteriophages’ genetic recombination and their hosts. Determining if a bacteriophage is lytic or lysogenic is fundamental in the field of medicine.
References
- Morrisette T, Kebriaei R, Lev KL, Morales S, Rybak MJ. Bacteriophage Therapeutics: A Primer for Clinicians on Phage‐Antibiotic Combinations. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy. 2020;40(2):153-68.
- Álvarez B, Biosca EG. Bacteriophage-based bacterial wilt biocontrol for an environmentally sustainable agriculture. Frontiers in plant science. 2017;8:1218.
- Joy JP. Exploring the lytic and lysogenic life cycles of bacteriophages. CourseSource.
- de Jonge PA, Nobrega FL, Brouns SJ, Dutilh BE. Molecular and evolutionary determinants of bacteriophage host range. Trends in microbiology. 2019;27(1):51-63.
- Karen Steward PhD. (2018). Lytic vs Lysogenic – Understanding Bacteriophage Life Cycles. From Technology Networks; Technology Networks. Web.
- Harrison E, Brockhurst MA. Ecological and evolutionary benefits of temperate phage: what does or doesn’t kill you makes you stronger. Bioessays. 2017;39(12):1700112.
- Segall AM, Roach DR, Strathdee SA. Stronger together? Perspectives on phage-antibiotic synergy in clinical applications of phage therapy. Current opinion in microbiology. 2019;51:46-50.
- Valente et al. Isolation and characterization of bacteriophages from the human skin microbiome that infect Staphylococcus epidermidis. FEMS Microbes. 2021;2.
- Barrick Lab. (2020). Barrick Lab :: ProtocolsPhageTiters. Barricklab.org. Web.
- Jobling MG. Lysogeny of Escherichia coli by the obligately lytic bacteriophage T1: not proven. Mbio. 2021;12(3).
- Tan JA, Christie GE. Lysogeny. eLS.:1-1.
- Yahara K, Lehours P, Vale FF. Analysis of genetic recombination and the pan-genome of a highly recombinogenic bacteriophage species. Microbial genomics. 2019;5(8).