Introduction
Thermal expansion is a concept in physics that refers to a matter’s tendency to change its density, volume, or shape in response to the change in the surrounding temperatures, not including the passé transitions. Besides, it can also be defined as a generalized increase in the volume of a given material as its temperatures increase. Thermal expansion is expressed as a fractional change calculated by a change in temperature divided by a change in length multiplied by the change in the exact length measurement. The near zero expansion in physics is because of the binding of Zr (Fe)-O-V chain. This paper focuses on the materials that have near zero thermal coefficients.
Condensed state physics science is the field of physical science that mainly deals with microscopic and macroscopic physical matter properties. Primarily, the area deals with liquid and solid phases that arise from the electromagnetic forces between atoms. In physics, near zero thermal coefficients (NZE) is a unique physiochemical process in which some of the materials mainly contract when heated. The criterion is different from the standard materials because the experimentalist expects a material to expand when heated and contract when cooled. However, this material goes on the vice versa. However, the materials that undergo a near zero thermal expansion have potential engineering, electronic, photonic, and structural applications. Near zero thermal expansion coefficients may not be permanent to most fabrics but occurs in materials such as water when nearing its freezing point (0-4 degrees Celsius), silicon at shallow temperatures, and cubic zirconium tungstate at temperatures exceeding 1000 degrees Celsius. NZE is important because it enables ice to float on water preventing the continental part of the crust from sinking in its oceanic position.
Physical principles behind the materials
As mentioned, NZE materials mainly offer a tremendous opportunity for essential and effectively applied research. However, the critical origin remains challenging to be understood by learners. The materials have both electrical and magnetic properties, which can be presented through an experiment. The calculation is termed to be the first fundamental principle of the materials. The material used in the A-site ordered perovskite SrCu3Fe4O12 which directly clarifies its non-zero mechanism. The stated material is an antiferromagnetic metal. Its magnetic ordering can be demonstrated as the C-type within the iron ions of the mixed-valence B site, which has paramagnetic copper ions at the earlier A-site.
Concerning the electronic structure analysis of the materials as a principle, it is revealed that some temperatures induced copper ions and iron interstice charge transfer that gets mediated by the corner that shares the identified O atoms. The magnetic interaction of the material mainly undergoes a transition that results in ferromagnetism from antiferromagnetic. From the phonon calculations, it is clear that the material mentioned is thermodynamically stable at both high and low temperatures. However, some phonon branches appear to have degenerated, indicating that it is easy for the material to transfer some energy between the two modes. The two modes are high and low temperatures, making the material qualify in the class of low thermal expansion. From the discussed principle, we find that significant near-zero thermal expansion of Zn4B6013 is mainly from the intermetallic charge induced by a place’s hotness or coldness. Finally, the change providing the principal of the NZE material discussed is the intermetallic transfer caused by temperatures that relax the Sr-O and Fe-O bonding units with their respective oxides.
Additionally, the other fundamental principle of the materials is that they contract when heated and expand when cooled. At the microscopic and molecular levels, individuals can achieve the point by using molecular, microscopic levels. The experiment of a metal sphere passing through a ring is the best to show that most materials expand when heated. However, vice versa happens with heating and cooling. However, the knowledge of thermal expansion can be explained using the understanding of interatomic spaces in a material. Suppose an individual considers the size of a lattice crystal proximal to absolute 0 with its atoms located to each other. In that case, the particles will be vibrating slowly to some extent. As temperatures increase on the material or its surrounding environment, the mobile atoms vibrate more and later correspond to the existing materials increasing in size as more volume is taken by the vibrations. They discussed prices are different when it comes to the experiment of a negative thermal expansion. The higher the temperatures, the lesser the number of oscillations of atoms in the material. As a result, it finally leads to contraction, unlike an increase in ordinary materials like aluminum, steel, zinc, and many others.
Unique properties of NZE materials
Contextually, the materials are interesting to study because they go against the physical understanding of thermal expansion. The contract on heating while they expand on cooling. In simple terms, these materials increase in size on cooling but reduce in size when heated. The materials have some physical properties, which gives them the discussed characteristics. The materials have strong bonds between their atoms which results in low coefficients of thermal expansion. NZE has a substantial isotropic thermal expansion that ranges from 20-425k. The most exciting part about the materials is that individuals can use them to control the overall thermal expansion of other essential composites.
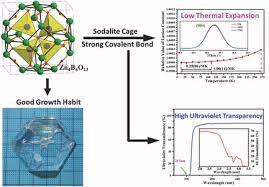
Similarly, when a harmful thermal expansion material is mixed with the typical material that expands on heating, one will realize that the negative may be used as a ram expansion compensator for the standard material. There may be the formation of composites that are tailored with zero thermal expansions. The latter material is usually observed in a closely packed system with directional interactions and other complex compounds like Zn4B6013.
Generally, one can be interested in the materials because they exhibit different behavior than the standard thermal expansion materials. Besides, the materials can be applied in different feels implying that they are essential. For instance, they can help form a composite of another material that has a positive thermal expansion, as mentioned. It can easily permit altering the stated thermal increase of the available compounds or having other composites with thermal additions closer to zero. As a result, the positive and negative thermal expansive materials reimburse each other to accept a certain targeted amount if their temperatures are changed. The materials mentioned are accommodating in the engineering profession in which there exists a necessity for having materials with CVTE that is relatively close to zero. For instance, it is helpful in precision instruments application.
Additionally, the glass-ceramic cooktops inclusion of Ceram requires temperature gradients that are significant and effective swift changes in temperatures while cooking because only specific cooktops will get heated when one is cooking. As a result, it is crucial to maintain the temperatures because the temperatures at one point may result in the breaking of galls due to the law of pressure. Finally, life instances displaying the necessity for materials that have tailored thermal expansion are dental guts. For example, if the fillings try to expand more than the teeth, it may cause toothache. If the mentioned dental fillings are made of a combined positive and negative thermal expander, then the overlap expansion can be tailored to protect the tooth enamel.
The latest research of such materials
According to research, industrial technology has developed, and there is sufficient study of the new technology involving the mentioned aspects. Various harmful thermal expansion materials have been developed in the last decade to add on the most common water when cooling (1-4 degrees Celsius). The discovery of more extensive isotopic NZE materials has advanced the research on expansion. It derives directly from its characteristic structure that is classified as conventional NZE. Additionally, the materials that have been organized in that record have increased rapidly. Besides, NZE made remarkable progress in the transition phase-type by use if the phase accompanies transition. The transition has been accompanied by volume contractions when they are heated. The stated imported materials have established a paradigm shift that is in control of thermal expansion.
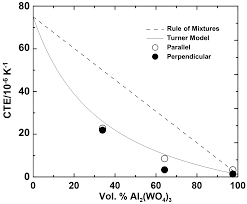
After discovering Zn4B6013 in 1996, scientists have come up with the latest giant NZE acting as the improvement material. The successive discovery of the mentioned material has encouraged improved and remarkable research progress during the last decade in physics. The changes have brought about a paradigm shift that has enhanced the thermal expansion process’s total control in the current technology. The new technology has classified the NZE materials into phase transition type and conventional thermal expansion. For instance, the current decade is discussing some of the latest examples of NZE. In stark contrast to usual materials, some materials compress when heated. The silicon oxide family, which included -eucryptite (LiAlSiO4) and cordierite (Mg2Al2Si5O18) and was discovered in the 1950s, is one example. The silicates possess strong covalent bonds, such as Al–O and Si–O ties, and weak ionic bonds, such as Li–O and Mg–O connections. Ionic bonds expand when heated, leading to the formation of double dimensional sheets in -eucryptite.
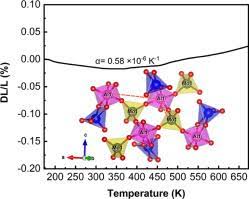
The thermal growth of these ionic unions pushes these double dimensional pieces closer since they are interconnected by solid covalent connections that less expands when heated. When eucryptite with a hexagonal crystal structure is heated from 293 K to 1073 K, the in-plane direction with the significant Li–O bond expands by 0.62 percent, while the out-of-plane surveillance with the dominating Si–O bond contracts by 0.62 percent. Forming a combined aspect with an NTE quantifiable as a thermal-expansion compensator is a well-organized way to manage a material’s thermal expansion. Numerous commercial materials are available, including zero-expansion glass for exactness optical tools and a zero-expansion porcelain material for semiconductor ploy manufacture. Many previous investigations have looked at composites using NZE minerals, specifically -eucryptite, as thermal expansion compensating fillers. Composites containing Mn3AN have been reported as phase-transition-type gigantic NZE materials. Because their effective chemical reactivity at higher temperatures is more significant than standard NZE materials, phase-transition-type NZE tools are challenging to use as compensating fillers for TE, especially in Metal Matrix Composites (MMCs).
Techniques used in tuning materials to lower thermal expansions coefficients to near zero or negative. Some methods used to turn materials into lower expansions include interatomic bonding, shown in figure 1. It offers variations of energy at rest with the interatomic distance for each pair of atoms that have been bonded. The vibrational energy gets quantified with an increase in temperatures. The occupation of the exceptional higher levels directly leads to the slight increment in the mean interatomic distance, always relative to the minimum energy at equilibrium. It could happen due to a rather sharp first-order phase transition between two separate states or gradually as the temperature changes. Although there are many NZE materials, individuals may categorize them into two groups based on their inherent mechanisms.
Electronic NZE is instigated by a decrease in distance of the first neighbor because of changes in interatomic probability due to heating. These changes can result from numerous physical property transitions that modify electron density distribution, including superconductivity, charge transfer, Ferroelectricity, and magnetism. Many framework-type materials with a high proportion of double connected linkers display structural NZE, which results from a decrease in second- or higher-neighbor distances because of dominant effects from crosswise vibrations, including tensional or bending modes. If the methods are established in many framework types, materials with a high percentage of 2-connected linkers exist. In nanoparticles, the intrinsic NZE procedures may be advanced, most likely due to electronic special effects resulting from the localization of well-ordered or correlated states closer to the surfaces. The amount of interior voids is reduced when bulk artifacts are heated, which results in different morphological processes for NZE. Ceramics have a microstructural NZE.
Examples of near-zero thermal coefficient materials and figures of their microstructures
As mentioned, most materials tend to expand when heated and contract when cooled, leading to thermal stress and even failure to some extent. Thermometric materials mainly exhibit negative thermal expansion, implying that they react vice versa to the standard materials with a near-zero coefficient thermal expansion. The key examples are polymer ceramic materials used simultaneously to optimize thermal and mechanical properties—the first example is INS Ceramic scaffolds, prepared by the freeze casting method. The material is written as Al2W3O12 and has a coefficient of 2 × 10−6 K−1.
Practical applications for low thermal expansion materials
Individuals can apply common thermal expansion materials such as silicon nitride, silicon carbide, and diamond in various ways. Silicon nitride is used in hybrid ball bearings together with ceramic balls and steel races. Besides, Silicon Nitride is used in machine tool spindles, vacuum pumps, and sterilizing lubricated dental drills.
Uses of Silicon Nitride
Components of a Reciprocating Engine
The most significant market for silicon nitride components for combustion and wear parts is reciprocating (diesel and spark-ignited) engines. Their growth has proven to be a more complex and challenging process than anticipated. Cost issues and the difficulty of mass manufacturing sophisticated ceramic components have stifled expansion, but the material has also been hampered by design conservatism and worries about ceramic component dependability. All ceramic bearings are utilized in settings where metals are prohibited due to corrosion, electric, or magnetic forces—for example, seawater attacks on tidal flow meters or electric field seekers.
Bearings
Fully dense silicon nitride improves the performance of lubricated roller and ball bearings at high temperatures because of its wear resistance, low friction, and high stiffness. HPSN bearings outperform traditional higher-density steel and stiff metal bearings in bearing life, speed capabilities, and corrosion resistance. Machine tool spindles, vacuum pumps, and sterilizable and non-lubricated dentistry drills are all examples of applications.
Working with Metal
Sintered silicon nitride’s hot hardness, fracture toughness, and thermal shock resistance allow it to cut cast iron, hard steel, and nickel-based alloys at speeds up to 25 times faster than traditional materials like tungsten carbide. Silicon nitride-based cutting tools are now widely utilized in the automotive and aerospace sectors to mill cast iron and nickel superalloys. Silicon nitride cutting tools have a present market worth of around $50 million per year. Still, because they are not suitable for machining high silicon aluminum alloys, their future growth will be limited as the car industry shifts to aluminum rather than cast iron blocks.
Applications in Industry
learners can use the material qualities in a wide range of typical industrial applications. Because the working conditions are less demanding than in the previous applications, reaction bonded silicon nitride (RBSN) is frequently employed.
The following are some examples of applications
Silicon nitride is finding a rising demand for non-automotive wear components. Fixtures for positioning and transferring metal pieces during operations like induction heating and resistance welding, for example, make use of the material’s electrical insulation, wear resistance, low thermal conductivity, and thermal shock resistance.
Handling molten aluminum, zinc, tin, and lead alloys with spouts, nozzles, thermocouple sheaths, and melting crucibles. Metallic components are becoming less appealing as the need for regulated metal purity grows.
Applications in the Future
If silicon nitride progresses from a niche to a critical industrial material, it must experience significant expansion in an application. This approach necessitates a lower cost and better or at the very least maintained characteristics and dependability. Engine technology is the sector that is most expected to gain from future growth, as lower fuel usage and emissions become increasingly important drivers. Surprisingly, the material has yet to be widely employed in gas turbine engines (the initial reason for its invention), owing to the limited availability of small to medium-sized turbine engines. The use of diamonds is well-known in a variety of sectors.
Diamonds are well-known not only for their beauty but also for their practical use. It is utilized everywhere, from petrol stations to mines to being regarded as a valuable gem that we wear on our bodies.
Jewelry with diamonds
Most individuals are aware that they use diamonds to make expensive jewelry. The diamond stones have a stunning sparkle, making them a popular principal point of interest in numerous jewelry designs and a significant and influential revenue source for the nation’s economy. When it comes to engagements or weddings, diamonds rings are often used as the first option as jewelry gifts.
Similarly, diamond is an extensively utilized metallic substance in industries besides its role as jewelry. From research, diamond is the most multifaceted metal on the earth, and it transmits heat quite affluently. In industries and factories, both its synthetic components are used as a raw materials in manufacturing resources. The jewels created in laboratories have qualities comparable to actual stones, but the chances of getting them are few. Furthermore, natural ornaments do not take after true treasures, and only a minute portion of them may be misused by enterprises. People can find some in the form of “bort” and small diamond remains and dust.
Automobile Manufacturing
Diamonds are also employed in the manufacture of automobiles. Every high-tech vehicle has 1.5 carats of diamonds. Drill bits and other components are utilized in the construction of cars, and they are an essential element of the process.
Conclusions
Thermal expansion is a concept in physics that refers to a matter’s tendency to change its density, volume, or shape in response to the change in the surrounding temperatures, not including the passé transitions. Additionally, the other fundamental principle of the materials is that they contract when heated and expand when cooled. However, some phonon branches appear to have degenerated, indicating that it is easy for the material to transfer some energy between the two modes. The two modes are high and low temperatures, making the material qualify in the class of low thermal expansion.
From the discussed principle, we find that significant negative thermal expansion of Zn4B6013 is mainly from the intermetallic charge induced by a place’s hotness or coldness. In simple terms, these materials increase in size on cooling but reduce in size when heated. The materials have some physical properties, which gives them the discussed characteristics. The materials have strong bonds between their atoms which results in low coefficients of thermal expansion. NTE has a substantial isotropic thermal expansion that ranges from 20-425k. The most exciting part about the materials is that individuals can use them to control the overall thermal development of other essential composites. Composites made of a freeze-cast thermometric ceramic, Al2W3O12, infilled with PMMA, were made and described. Lamellar scaffolds were successfully constructed using the right ceramic particle size and dispersion in the slurry and the right freeze-cast product sintering temperature. These were filled with PMMA polymer, and the results show that individuals may utilize this process to make composites with variable thermal expansion. The inclusion of PMMA and the lamellar microstructure improved the material’s resistance to micro-crack propagation in the direction perpendicular to ice formation, implying that instructors enhanced the material’s resistance to micro-crack propagation in that direction. Further development of the freeze-casting technology as applied to thermometric ceramics might give a mechanism to compensate for the mechanical qualities of materials with low or near zero thermal expansion that are innately poor.
References
Gao Q, Wang J, Sanson A, Sun Q, Liang E, Xing X, Chen J. Discovering large isotropic near-zero thermal expansion in framework compound AGB (CN) 4 via the concept of average atomic volume. Journal of the American Chemical Society. 2020;142(15):6935-9.
Guan QF, Yang HB, Han ZM, Zhou LC, Zhu YB, Ling ZC, Jiang HB, Wang PF, Ma T, Wu HA, Yu SH. Lightweight, challenging, and sustainable cellulose nanofiber-derived bulk structural materials with low thermal expansion coefficient. Science advances. 2020;6(18):eaaz1114.
Hao H, Xu S, Jin N, Wang M, Wang Z, Yang L, Chen J, Wang G, Wang G. Negative thermal expansion material: promising for improving electrochemical performance and safety of lithium-ion batteries. The Journal of Physical Chemistry Letters. 2021;12(26):6134-42.
Qi K, Yang Y, Hu G, Lu X, Li J. Thermal expansion control of composite coatings on 42CrMo by laser cladding. Surface and Coatings Technology. 2020;397:125983.
Shen Y, Wang FQ, Wang Q. Ultralow thermal conductivity and negative thermal expansion of CuSCN. Nano Energy. 2020;73:104822.
Shi N, Sanson A, Gao Q, Sun Q, Ren Y, Huang Q, de Souza DO, Xing X, Chen J. Substantial near-zero thermal expansion in a low-cost and facile oxide of Cu2P2O7. Journal of the American Chemical Society. 2020;142(6):3088-93.