4-chlorophenoxyacetic acid
As it is known, plants are capable of unlimited growth throughout life, which is mainly due to the peculiarities of the molecular organization of cells. In particular, the main growth hormone of plants belonging to the group of auxins is indoleacetic acid, whose structure is shown in Fig. I. Human agricultural activity determined the demand for high production volumes, which, in turn, led to in-depth research in the field of artificially synthesized alternatives for indoleacetic acid. Although most of the analogs could reasonably copy the physiological actions of the target molecule, special attention should be paid to the three main compounds with high auxin activity. These are 4-chlorophenoxyacetic acid (Fig. II), 2,4-dichlorophenoxyacetic acid, 2,4-D (Fig. III), and 2,4,5-trichlorophenoxyacetic acid, 2,4,5-T (Fig. IV). Moreover, the biological effect of 2,4-D and 2,4,5-T is so great that it produces irreversible plant growth, causing its death. This phenomenon has been used to create herbicides for some broad-leaved plants, although recently it was found out that 2,4,5-T forms a highly toxic compound, namely 2,3,7,8-tetrachlorodibenzo-p-dioxin, TCDD or dioxin, (Fig. V), and therefore it has been prohibited to apply it.
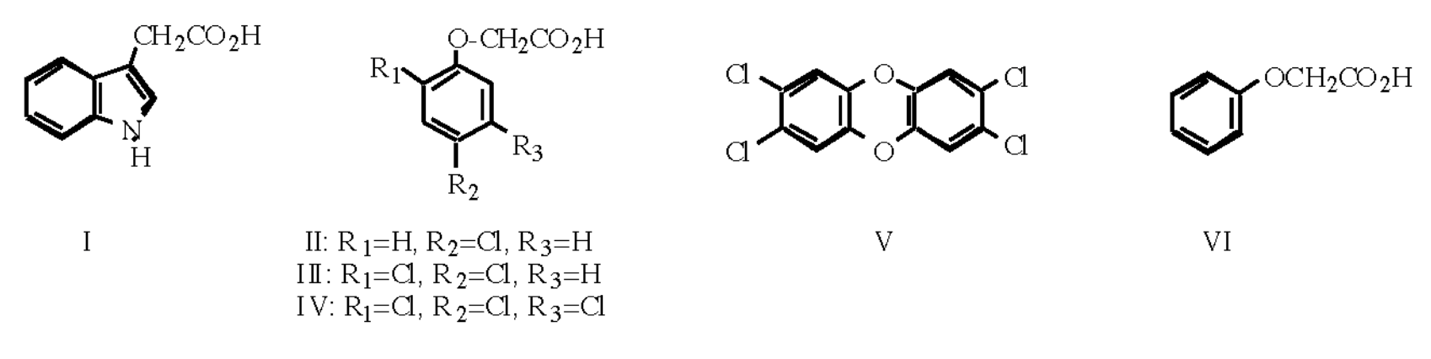
It is fair to say that 4-chlorophenoxyacetic acid, similar to another auxin analog, namely 2-naphthoxyacetic acid (Fig. VI), has a noticeably lower biological effect on plant growth than 2,4-D and 2,4,5-T. Nevertheless, their use is very common in the agricultural sector. For instance, in low concentrations, 4-chlorophenoxyacetic acid and 2-naphthoxyacetic acid can be used as a means to prevent premature fall of fruits, stimulate parthenocarpy, and promote root development in cut plants or vegetative growth. A wide range of practical use of these analogs dictates their high commercial value for the producer, including if the conditions of fruit-bearing plants do not meet the expectations of agronomists.
The chemical synthesis of 4-chlorophenoxyacetic acid is realized through the reaction of nucleophilic displacement between anions of the 4-chlorophenolate anion (Fig. VII) and chloroacetate (Fig. VIII), the product of interaction of which is subjected to acetic acid treatment.
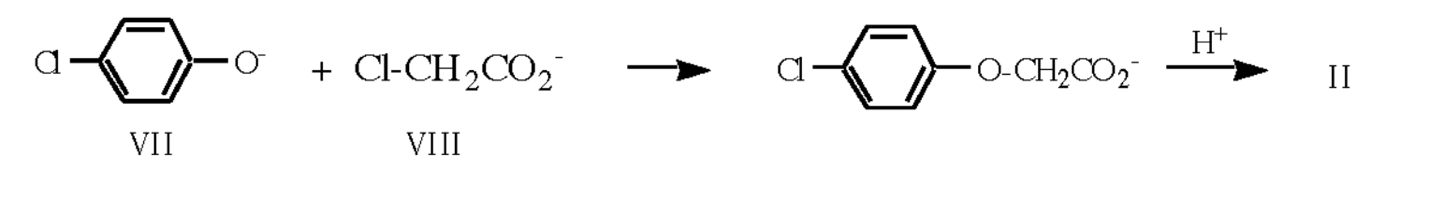
The utility of traditional 4-chlorophenol for this synthesis can be complicated by several factors, among which are the causticity, toxicity, and difficult recyclability of the reagent. In this case, it is reasonable to replace 4-chlorophenol with an analog having a similar molecular structure. Thus, the synthesis of 4-chlorophenoxyacetic acid should be preceded by the reaction of the formation of a mixture containing 1.0 M sodium 4-chlorophenolate and 2.5 M sodium hydroxide. The addition of chloroacetic acid to the prepared solution allows obtaining chloroacetate ion (Fig. VIII).
Experimental
Sodium 4-Chlorophelolate (Fig. VIII) — Sodium Hydroxide Solution
The researchers prepared a solution containing sodium 4-chlorophelolate and sodium hydroxide in two consecutive steps. First of all, to synthesize 2.5 M sodium hydroxide, they dissolved 140 g, which is equivalent to 3.5 moles, of substances in 800 mL of water, and the dissolution reaction, being exothermic, heated the reaction mixture. Then, they added 128.6 g, which is equivalent to one mole, of 4-chlorophenol to the obtained solution; after that, the reaction volume was brought up to the mark of 1 L. It should be remembered that the resulting solution had high causticity, and therefore the interaction with it should have been careful. Liquid penetration can cause skin lesions: in this case, skin hygiene under running water should be performed immediately.
4-chlorophenoxyacetic Acid (Fig. II)
Synthesis of 4-chlorophenoxyacetic acid was carried out by careful execution of five consecutive steps. First of all, a solution from the previous synthesis was carefully placed in a 125 mL Erlenmeyer flask, to which 9.5 g of chloroacetic acid, which is equal to 1 mole, was added. The initiation of the interaction was stimulated by heating in a steam bath (or a boiling water bath as an alternative) for half an hour, after which the solution was cooled for a few minutes. Then, 40 ml of 6.0 M solution of hydrochloric acid was added to the standing solution, which led to the formation of a white crystalline precipitate. Mixing and subsequent cooling in an ice bath were used to stimulate the completeness of the reaction and produce pure sludge. The solid matter was isolated by suction filtration and was washed under cold distilled water. Recrystallization of 20 mL of a solution containing water and ethanol in ratio 1:2 correspondingly allowed to obtain 5 g, which is equivalent to 53% yield, of large white crystals. The following physicochemical parameters were identified in their study: (i) mp 155-157 (lit 156-7); (ii) IR (on KBr) 3200-2300, 1710, 1600, 1480, 1430, 1230, 1070, and 810 cm-1; (iii) NMR (on acetone-d6) δ 4.68 ppm (s, 2H), 6.92 ppm (d=9.5 Hz, 2H), 7.3 ppm (d, J = 9.5 Hz, 2H), and 7.77 ppm (s, 1H).
References
Wood, W. F. (1981). p-Chlorophenoxyacetic acid-preparation of a synthetic plant hormone. Journal of Chemical Education, 58(1), 76-77.