Abstract
Background
HIV drug resistance is becoming a major concern not only in developing nations but also in developed countries such as the United States, Canada, the United Kingdom, and Germany. The prevalence rate of the condition is high in Sub-Saharan Africa. The focus of this study was to investigate the possible causes of the resistance, its prevalence, and ways of dealing with the problem.
Methods
The review of literature focused on primary sources, especially peer-reviewed journals. The journals were obtained from online databases such as PubMed Central, the National Center for Biotechnology Information (NCBI), the Online Mendelian Inheritance in Man Database (OMIM), and WebMed. Other important sites used include Medscape, MedicineNet, EMedicine, EBSCO Information Services, and ProQuest
Results
The review revealed that enzyme mutation in the genetic structure of HIV is the primary reason that causes drug resistance. In cases where one is infected with both HIV-1 and HIV-2, it may pose challenges to the effective treatment of the virus. Factors such as poor adherence to medication, the inability of the body to absorb the drug, and varying pharmacokinetics were identified as some of the leading factors that cause drug resistance.
When the condition of an HIV-positive individual continues to deteriorate even when they are under medication, it is recommended that such an individual should be taken through phenotypic and genotypic testing to establish if the virus has developed some form of resistance. The use of highly active antiretroviral therapy (HAART) would be appropriate in managing such unique conditions.
Conclusion
The findings of this study show that cases of HIV drug resistance are on the increase, especially in parts of Sub-Saharan Africa. To help overcome this problem, it is recommended that patients should avoid cases of reinfection. Patients who have developed resistance to the drug would need to use HAART, which is a more aggressive and effective form of managing the condition.
HIV
The use of antiretroviral (ARV) treatment has given hope to millions of HIV (human immunodeficiency virus) patients across the world. The medication helps to suppress the replication of the virus in the body and strengthens the immune system. Recent studies show a worrying trend of cases of drug resistance among HIV patients. According to research by Tang and Shafer (2012) among HIV patients in Africa shows that the virus sometimes mutates, making it difficult to manage the medication using conventional ARV therapy.
Factors such as lack of viral load monitoring, cases of interruption of the medication, and the inability of the body to absorb the drug are known to cause resistance to ARV medication (Jespersen et al., 2015). Patients who have both HIV-1 and HIV-2 viruses may not respond well to medication. Some studies suggest that Africans tend to develop the K103N NNRTI mutation, which is often caused by poor adherence to medication (Rhee et al., 2015). In this case, genetic polymorphism slows plasma MNRT clearance because of the interruption of the medication.
When a patient develops resistance to the ARV drugs, the consistent depletion of CD4+ T cells cannot be stopped, which means that the patient would have a severe immune system dysregulation (Onywera et al., 2017). The condition of the patient would progress rapidly into AIDS. When a patient fails to respond to cART regimens, they become susceptible to opportunistic diseases such as renal complications, cardiovascular diseases, liver problems, malaria, and diarrhea among others.
It is estimated that there are over 36 million HIV-positive patients around the world, most of who are in Sub-Saharan Africa. A significant number of these people are breadwinners in their families. The only hope that they have to lead a long normal life is when they get the right medication that would suppress the duplication of the virus and strengthen their immune system. As such, HIV drug resistance is a major concern that needs the attention of medical experts and other stakeholders in the field of healthcare. In this research paper, the focus is to understand the possible causes of HIV drug resistance among patients and steps that can be taken to address the problem.
Methods
When conducting this research, it was necessary to collect data from reliable sources. According to d’Ettorre et al. (2014), the primary essence of any research project is to address the existing knowledge gaps by providing new information or evidence. HIV is a controversial topic in the field of medicine. A report by Restrepo et al. (2019) shows that about 940,000 people died of AIDS around the world in 2018. Another 36.9 million people are living with the condition. It is necessary to find ways of managing this global pandemic. As such, it was necessary to find credible information that would guide the researcher to make a conclusion based on facts.
The problem is not unique to the United States but also in other parts of the world. It is a more serious problem in Sub-Saharan Africa than it is in the United States and other developed nations in Europe. When conducting this study, it was desirable to collect primary data from healthcare institutions and individuals who have been using antiretroviral therapy.
However, geographic barriers and limited time available for the research made it impossible to collect data from these respondents. The researcher couldn’t travel to Africa and other worst affected areas to gather data because of the geographic barrier. As such, the researcher relied on publications made by other scholars.
Online medical databases were used to identify the resources needed for the study. PubMed Central and the National Center for Biotechnology Information (NCBI) provided important journal articles, which were instrumental in this investigation. The Online Mendelian Inheritance in Man Database (OMIM) and WebMed also proved crucial in providing the information needed in this project.
Other important databases used include Medscape, MedicineNet, EMedicine, EBSCO Information Services, and ProQuest. The researcher used keywords such as HIV-1/2 dual infection, antiretroviral treatment, drug resistance, HIV prevalence, and CD4 cell. The information obtained from these sources was used to inform this project.
Discussion
According to Zoufaly et al. (2014), drug resistance refers to a condition where a disease-causing organism, which in this case is the HIV virus, to continue replication despite the administration of a drug that is known to destroy it. The body of such a patient would not respond to the ART treatment. One of the primary causes of HIV drug resistance is the mutation of the virus. The virus changes its genetic structure, especially enzymes in the virus, which enable it to replicate. Other than mutation, which is often caused by poor adherence to the medication, the presence of HIV-1 and HIV-2 in a patient may also cause resistance.
It is not common to have cases where one has to administer two types of ARV treatment to a patient. It means that when one is infected with both types, the medication will only focus on managing one of the types of the virus, which means that the other can easily mutate in response to the medication. In this section, it is necessary to look at socio-economic factors that would lead to mutation, investigate the scientific concept of HIV drug resistance, and factors that can be taken to address this medical problem.
Socio-Economic Factors Associated with the Spread of HIV
When analyzing HIV resistance to antiretroviral drugs, it is also important to look at factors that often lead to cases where an individual is infected with more than one type of the virus. According to Nakanjako et al. (2011), one of the leading factors that make it difficult to manage the viral load among some of the HIV-positive people is the existence of both type HIV-1 and HIV-2. Such cases are common when an individual who is already infected with one type is re-infected with a different type of the virus.
Managing the condition of such a patient may be very challenging. In this section, the researcher will look at socio-economic factors associated with the spread of HIV in Sub-Saharan Africa where the prevalence of this pandemic is highest in the world.
Poverty
According to Soria et al. (2011), poverty is one of the leading causes of the spread of the HIV virus in Africa. South Africa is one of the most populous African countries. However, unemployment among youths is a major problem that the government is struggling to address. Most of the poor youths end up in slums where they engage in irresponsible sexual practices as a means of earning income to sustain their basic needs.
In Kenya and other East African countries such as Tanzania, sex tourism is a booming business (Chan et al., 2016). One of the factors that have promoted this practice is the poor implementation of laws relating to prostitution. The biggest challenge associated with sex tourism is that it involves children, some as young as eleven years. At such a tender age, these young boys and girls do not know much about safe sex.
Both local and foreign visitors use these minors by giving them financial benefits in exchange for sex. They are constantly exposed to HIV infection every time they engage in such irresponsible practices. Lee, Wong, Wong, Wong, and Chan (2017) argue that the problem is that the extreme level of poverty in these regions leaves the affected group with no alternative other than to engage in prostitution. Some of them understand the dangers of unsafe sex but they know it is the only reliable source of income. It is the only way that they can avoid starvation.
Myths and misconceptions
Scholars have cited misinformed beliefs as some of the leading factors that increased the prevalence of HIV among sections of Africa and other developed nations. According to He et al. (2016), there is a widespread belief among various African communities that the only way of being cured of HIV is to have sexual intercourse with a virgin, most preferably a very young girl. As such, fathers, uncles, brothers, and close family friends are turning against young girls who trust them.
They rape these girls believing that doing so will cure them of their condition. It is important to note that the rapist knows well that he has the virus, and in so doing, he transfers it to the victim. The problem is compounded by fear among the victims who fail to report the crime as soon as possible so that they can get medication. Some of them would be subjected to repeated sexual abuse before they can be rescued. Most of them get to the hospital when it is too late to be given the Pre-exposure prophylaxis (PrEP) that can protect them from the virus.
Merci et al. (2017) explain that some women in the region are also embracing the belief that having unprotected sexual intercourse with young boys would help reduce their viral load. They entice these unsuspecting boys and young men with financial benefits.
Traditional practices
Some of the traditional African practices have also been cited as a major cause of HIV transmission. Wife/widow inheritance, a practice where a woman is required to get married to her late husband’s brother or cousin soon after the burial, is common in many African tribes. According to Mahajan et al. (2010), wife inheritance is common among the Luo of Kenya and Uganda, Dinka of South Sudan, various communities in Ghana and Nigeria, and parts of Malawi and South Africa.
The problem is that HIV prevalence in these regions is high, which means that some of these men die of the virus. Their infected women would then transmit the virus to the inheritor, who will then infect his wife. The vicious chain would continue, making it difficult to control the pandemic. Traditional circumcision is another common practice that may expose healthy youths to the virus. When the same knife is used to circumcise several boys or girls, then it is possible that the virus can be transferred from one of the initiates to another.
Social stigma
Social stigma is another major concern, in not only Sub-Saharan Africa but also other parts of the world. Philpott et al. (2004) argue that social stigma makes it difficult for a person to open up about being HIV-positive. There is a general perception that anyone with the virus is sexually immoral. Ponnan et al. (2019) explain that the society in the western world believe that when one has the virus, then he or she can die at any time.
There is also the fear that such a person can spread the virus to healthy people not only through unsafe sex but also through contact. Friends and some family members would shun such people. To avoid being in such a predicament, many people opt to remain silent about their condition. Others even fear being tested because they feel they cannot deal with the situation when they are informed they have the virus. Late diagnosis of the disease makes it difficult to manage the condition. Such an individual would also end up spreading the virus to others during the period when they are unaware that they have the virus.
In some extreme cases, HIV-positive people would deliberately spread the virus to many unsuspecting individuals either as retaliation or as a way of making the condition common to a large number of people to reduce the stigma. Ponnan et al. (2018) believe that such practices are common in institutions of higher learning and in populated slums where rates of absolute poverty are high.
Crime
Cape Town, Durban, and Nelson Mandela Bay are ranked top African metropolitans with the highest rates of crime (An et al., 2002). It is not surprising that they also rank relatively high in reported cases of those who are living with the HIV virus. In Mexico, Acapulco and Tijuana are known for the high prevalence of crime. In these territories, the prevalence of HIV infection is also high. Duwal, Seeler, Dickinson, Khoo, and von Kleist (2019) explains that the problem is that in crime-prone cities, cases of rape and other forms of sexual abuse are common. Sex is the primary way of transmitting the virus from an infected person to a healthy individual.
Drug abuse is also common in these regions. In the United States, the city of Miami in Florida has the highest prevalence of HIV infection (Lee et al., 1998). It is not surprising that the city is also grappling with the problem of drug abuse among teenagers and young adults. Some of the instruments they use to inject some of the drugs can easily spread the virus from one person to another. Boliar et al. (2018) also explain that once one is high on drugs, they can easily engage in irresponsible sexual practices. They can be easily manipulated into sexual practices, which then expose them to infection.
Antiretroviral Therapy
Antiretroviral therapy, commonly known as ARV treatment is the standard approach to managing HIV. The drug helps the body by strengthening the immune system, lowering the viral load, and reducing the risk of an HIV-positive person to transmit the disease to a healthy individual. Fusion inhibitors that block the virus from entering and attaching itself to specific cells within the body are some of the aspects of ART therapy.
CCR5 blockers work by blocking the host cell receptors, which effectively limit the ability of the virus to attach to specific cells (Trifone et al., 2018). The process causes interruption of the life cycle of HIV in the early stages of development. On the other hand, gp120 inhibitors functions by binding to the GP 120 proteins that the virus needs to enable it to attach to healthy cells (Liu et al., 2014). It means that this medication makes it impossible for the virus to attack healthy cells. Finally, gp41 inhibitors block the viral gp41 protein, making it impossible for the virus to fuse with healthy cells (Gutiérrez et al., 2019). The ARV therapy works by systematically creating an environment within the body that limits the ability of the virus to spread to human cells.
When HIV enters the human cell, an enzyme known as a reverse transcriptase would facilitate the copying of the viral RNA into DNA. The viral DNA copy would get integrated into the cell DNA of the host (Moir et al., 2008). Medical researchers have developed various ARV drugs, commonly known as reverse transcriptase inhibitors to block the reverse transcription that would interfere with the process of virus replication.
The non-nucleoside reverse transcriptase inhibitors (NNRTIs), often known as non-nucleosides, binds itself to the HIV enzyme known as a reverse transcriptase that is needed to facilitate the replication of the virus (Nikolova et al., 2016). It effectively blocks the virus from making copies, hence managing the viral load in the body. Nucleoside reverse transcriptase inhibitors (NRTIs) mimic nucleotides, which are the building blocks of the DNA of the virus (Siewe et al., 2014). Once incorporated into the DNA of the virus, they inhibit its growth by blocking the process of reverse transcription.
According to Chevalier and Weiss (2013), integrase inhibitors, which is another common form of ARV therapy, prevents the virus from inserting the genetic material into chromosomes of the host. It is widely used to block the reproduction of the virus within human cells.
On the other hand, protease inhibitors limit the ability of HIV to construct protein components that it requires to assemble new viruses. The complex nature of HIV makes it necessary to use a combination of therapies that focus on eliminating the perfect environment that it needs to replicate and spread to various cells within the body. It is important to note that these different forms of medication only focus on inhibiting the duplication of the virus and its free movement from one cell to another.
Biological Basis of Drug Resistance
The ARV regimen is expected to work in different ways to limit the ability of the HIV virus to replicate and spread to other cells within the body, as discussed in the section above. However, medical experts have had to address cases where the patient develops resistance to conventional drugs used in managing the condition. According to Cagigi et al. (2009), drug resistance among HIV patients is primarily caused by the mutation in the genetic structure of the virus.
The change in structure alters enzymes, which help the virus to replicate. The standard medication would not work effectively on the virus once its enzymes are altered. The following are the main ways through which the body of a patient can develop resistance to HIV drugs:
Transmission of drug-resistant HIV
The golden rule in the management of HIV is for the infected person to maintain the intake of the ARVs. In the United States and many other parts of the world, when one is diagnosed with the virus, the standard practice is to put them on drugs. Continued intake of the drug over the years may result in instances where the virus develops resistance to some of the common drugs used in managing the condition.
If one is infected in any other way with a mutated virus from an HIV-positive person, the drug-resistant variant virus would be transmitted. The mutated strain of the virus cannot be managed using one or more conventionally used HIV regimens. Such patients would require highly active antiretroviral therapy (HAART) to help them manage their condition. It is estimated that about 5 to 20 percent of new infections in the United States involve the mutated HIV strain that is resistant to one or more HIV medication (Apostolova et al., 2015). The problem is more severe in Sub-Saharan desert, especially in South Africa where a large number of adults aged below 45 years are actively using ARV to manage their condition.
It is unfortunate that exact data about the number of those who are infected with drug-resistant HIV in these countries is not easily available because of limited research. However, Conway, Konrad, and Coombs (2013) believe that a significant number of those who are newly infected with the virus in these countries have a greater risk of acquiring drug-resistant HIV.
While using pre-exposure prophylaxis (PrEP)
The pre-exposure prophylaxis is a common drug used around the world by people who are HIV-negative but are at risk of being infected with the virus (Dellar, Dlamini, & Karim, 2015). One occupation may be a risk factor that makes it necessary to use the drug. Commercial sex workers and nurses who offer care to HIV-positive patients are at great risk of being infected with the virus.
In a discordant couple, the HIV-negative partner would risk being infected if they continue to have unprotected sex without proper measures being taken to lower the viral load and minimize the ability of the patient to transmit the virus. In the United States, the U.S. Food and Drug Administration approved Truvada as an appropriate PrEP for those who are at risk of being infected. As shown in figure 1 below, this drug forms a protective layer around T-cells, making it impossible for the HIV virus to penetrate. The virus would die after a short while if it is unable to penetrate the cell.
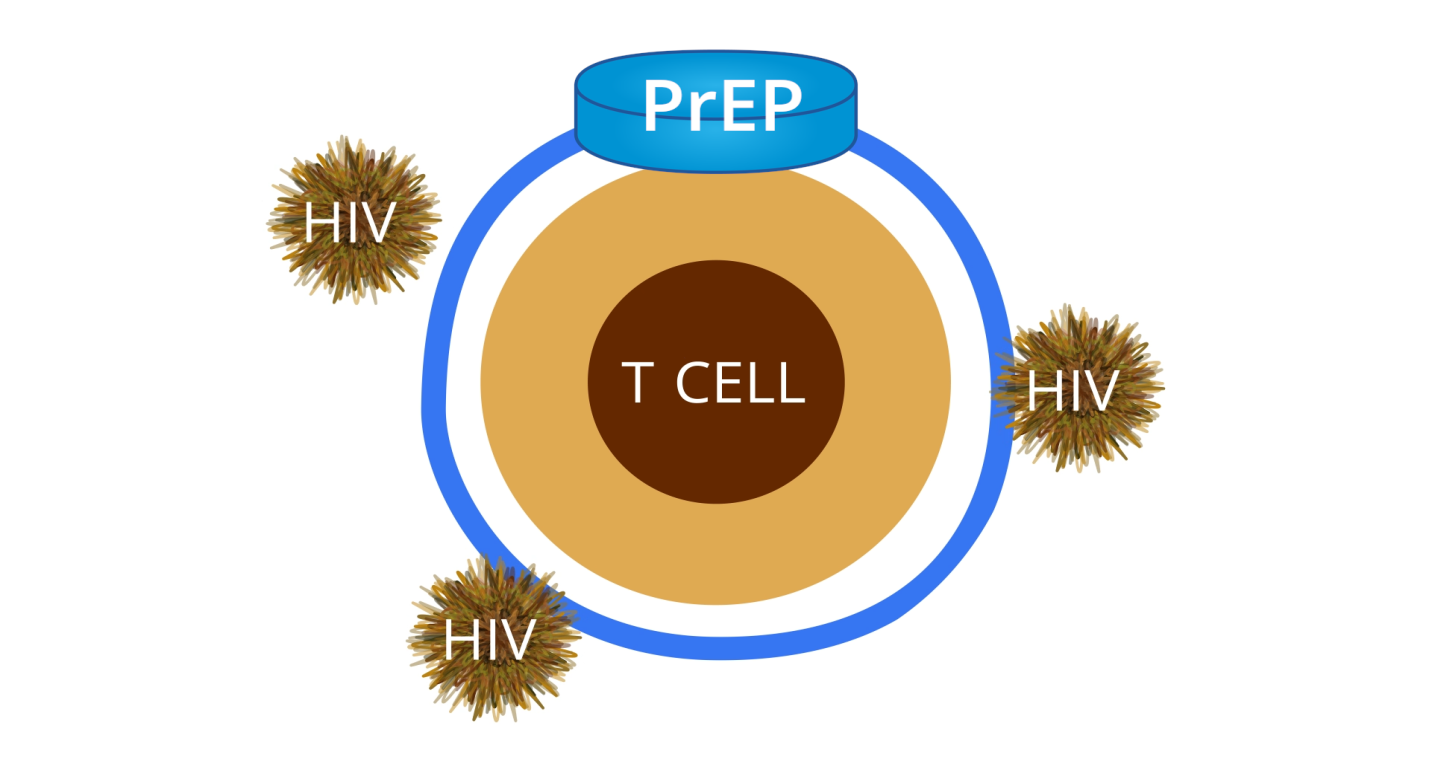
The protective layer can only be strong enough to prevent the entry of the virus if the patient adheres to the daily medication irrespective of whether they will be exposed to the virus or not. Duwal, Dickinson, Khoo, and von Kleist (2019) explain that the problem arises when some people fail to take PrEP as scheduled, only to resume a few days to the day they plan to engage in unprotected sex. In such instances, the strength of the protecting layer may be compromised, making it possible for the virus to penetrate the cell.
Such a patient would not realize that they are infected. When a patient continues to use PrEP when they are already infected, it causes resistance to HIV drugs, which use a similar approach to the PrEP to manage the condition. Duwal et al. (2018) argue that it is prudent for individuals who decide to use this form of protection to adhere to the instructions given to them to avoid cases of resistance. When one is aware that they were exposed to the virus, it is recommended that they should go for a medical check-up after three months just to ensure that they were not infected.
Factors That Affect the Effectiveness of HIV Regimen
The primary aim of ARV therapy is to lower the viral load within the body by inhibiting its reproduction and mutation (Dickinson et al., 2016). The regime also helps in delaying or preventing the mutation of the virus, making it easy to use conventional drugs to manage the condition. Unfortunately, there are cases where the medication fails to work as would be expected. The patient would be exposed to the adverse effect of HIV, which includes the progression to AIDS and the risk of death from various opportunistic diseases. The following are some of the factors that often affect the effectiveness of HIV drug medication:
Poor treatment adherence
When one is diagnosed with the HIV virus, one of the first steps that are often taken is to determine its type and the CD4 count. It helps in determining the type of medication that a patient should receive. When one is put on antiretroviral therapy, the primary goal is to inhibit the replication of the virus and the destruction of the white blood cells. The drug strengthens the immune system and ensures that the viral load is maintained at the lowest level possible. Skipping the ARV treatment allows the HIV virus to multiply within a short time. The virus will have the opportunity to destroy the immune system within the period when the patient fails to take the medication.
A weak immune system cannot effectively fight the virus, various other opportunistic diseases, and certain types of cancer (Duwal et al., 2016). Scientific research has also proven that poor adherence to HIV treatment increases the risk of drug resistance. The period within which the patient fails to take the medicine allows the virus to mutate in a way that makes it possible to fight ARV treatment. It means that when the patient resumes normal medication, the treatment may not work. The immune system will be too weak to fight the virus. The structure of the virus shall have changed in a way that makes it difficult to respond to the drug.
Poor absorption
The effectiveness of HIV drugs depends on the ability of the body to absorb it. Duwal and von Kleist (2016) explain that various factors may affect the process of absorption. Some people experience vomiting and diarrhea during the initial stages of taking the medication, which causes the drug to be expelled from the body before its absorption (Fletcher et al., 2014). Many patients are often not aware of what they need to do in case of intense vomiting and diarrhea. Excessive intake of alcohol may also impede the ability of the body to absorb the medication. Sometimes the body system may fail to absorb the medication because of genetic factors.
In such cases, the amount of HIV regimens in the body would be too low to fight the virus and manage its replication. Instead of having a positive effect suppressing the viral load, the medication will encourage drug resistance accumulation. If the problem of poor absorption is not addressed within a short period, the virus will mutate and that type of treatment will not work in future treatments. Grant et al. (2014) state that sometimes it is appropriate to take the medication with some supplement when it is established that the body is not absorbing the regimen as expected.
Varying pharmacokinetics
Once the drug has been absorbed into the system, it should be distributed, broken down, and removed from the body in the right quantity and at the right intervals. In some cases, the interaction between drugs may pose a serious challenge to a given patient. For instance, when NRTI is used alongside protease inhibitor Reyataz, Boliar et al. (2018) explain it is possible to have a case where that blood levels of Reyataz falls to very low levels, which may pose a serious challenge to the patient. Such a patient would require protease inhibitor Norvir to help in boosting the level of Reyataz in the bloodstream (Duwal et al., 2016). When the doctor fails to understand how to combine the right medication for the patient based on various factors discussed above, the drug may fail to function as expected.
Existence of different variants of the virus
Continuous use of antiretroviral drugs has also proven to contribute to cases of drug resistance among HIV patients. Restrepo et al. (2019) observe that a patient who adheres to the prescription of the ART can lead a normal life for several decades. However, the challenge that these patients face is that when they use a specific drug for a prolonged period, the virus would develop resistance towards it. It reaches a time when a given HIV regimen fails to manage the condition of the patient. In such a case, it becomes necessary to change the drug being used. It is necessary to ensure that the CD4 count of the patient is monitored closely to ensure that the medication is having the right impact. A consistent of a drastic drop in the CD4 count may indicate that the current drug is no longer having the right impact on the patient.
Managing Drug Resistance
The patient and the medical staff have the responsibility of managing the unique condition of a drug-resistant drug. It is necessary to look at two perspectives of managing the condition. On the one hand, it is necessary for those who are yet to have the drug-resistant variant to know how to manage their condition. On the other hand, those who have the variant resistant to medication should also know how to deal with the problem, as discussed below
How to avoid drug resistance
When one has an HIV variant that is resistant to drugs, their condition may become delicate. As such, it is important to take the necessary steps to avoid such a condition. Managing the condition requires a concerted effort by both the medical staff and the patient. One of the factors that an individual can take to avoid such a condition is to refrain from the misuse of PrEP (Duwal et al., 2016).
People who work or stay in environments where they are constantly exposed to the threat of being infected with the virus should know when and how to administer the drug. They should strictly adhere to the instructions given to them to ensure that they can realize the intended goals. Boliar et al. (2018) explain that responsible use of PrEP would require the relevant government department to promote awareness about the condition and effective ways of managing it.
Myths and misconceptions should be addressed in such campaigns to ensure that people are effectively empowered. Ponnan et al. (2019) argue that the use of PrEP should not be an excuse for an individual to engage in irresponsible and unprotected sexual practices with people known to have the virus. The society should also be reminded that HIV-positive individual risks being re-infected with other drug-resistant variants, which would complicate the treatment process. Some vengeful people often tend to engage in unprotected sex with other people as a way of spreading the virus, not knowing that they are exposing themselves to greater risks in the process.
When managing this medical condition, the HIV patient is encouraged to maintain a healthy lifestyle. They should engage in regular exercise, have a balanced diet, and avoid other viral and bacterial infections. A study by Restrepo et al. (2019) indicates that leading a healthy lifestyle is one of the best ways of managing the viral load in the body as low as possible. The CD4+ count will be at an optimal level and such an individual would lead a normal life. The benefit of effective management of HIV is that the virus will not mutate within a short period. It means that the same medication can be used for several years without having to change because of possible changes in the protein structure of the virus.
Understanding the HIV variant that one suffers from and administering the right medication is another important way of avoiding resistance. In some of the developing countries in Sub-Saharan Africa where the condition is prevalent, some non-governmental organizations are hiring semi-skilled individuals to help in administering drugs. Some of them are taken for 4-10 months of training before being posted to their workstations.
They do not understand how to determine the right medication for a patient based on the variant of the virus. They can make diagnostic mistakes or errors when dispensing drugs. The problem in these regions is that patients are either illiterate or semi-illiterate (Fletcher et al., 2014). As such, they would take the medication given to them without question. When the patient continues to get the wrong medication over a given period, the virus can develop resistance to drugs. Their health condition would continue to deteriorate even when they continue with their medication.
Dealing with drug resistance
When it has been established that an individual has an HIV variant that is resistant to drugs, it is appropriate to find ways of addressing the problem. According to Ponnan et al. (2019), one of the first steps that should be taken by the medical staff when the condition of the patient is not improving despite taking the medication is to conduct phenotypic and genotypic tests. These tests would help in determining the variant of the virus that is resistant to drugs.
Administration of highly active antiretroviral therapy is often recommended when addressing such a condition. When a given form of medication has proven effective, Restrepo et al. (2019) emphasize the need for the patient to adhere to the prescription to avoid further complications. They should be reminded of their delicate condition and the need for them to remain responsible for managing their condition. A healthy lifestyle is equally important for a person managing a condition where the virus is resistant to drugs.
Conclusion
Recent studies show that over 36 million people are suffering from the HIV virus around the world. One of the biggest challenges associated with this pandemic is that the virus can be transferred easily from one person to another through sex or the sharing of objects such as a syringe. Effective management of the condition minimizes the ability of an infected individual to transfer the virus to a healthy person. Antiretroviral treatment is often used to help in managing the condition.
The current concern that medical experts and stakeholders in the healthcare sector have to address is the emergence of an HIV variant that is resistant to commonly used ARV. Studies show that one can be infected with the drug-resistant drug. Continued use of the drug may also cause resistance within the body of an infected person. Irresponsible use of PrEP was identified as another factor that can cause resistance to some of the common HIV medications.
Social awareness is one of the best ways of dealing with the problem. People should be aware of the dangers associated with being infected with both HIV-1 and HIV-2. They should also understand the dangers of misusing PrEP when engaging in irresponsible sexual practices. When it is established that a given patient has developed resistance to some of the common HIV therapies, it may be appropriate to administer highly active antiretroviral therapy (HAART). Most importantly, it is necessary to find a lasting solution to this pandemic. Medical researchers should remain committed to finding a cure for this condition.
Reference
An, P., Nelson, G. W., Wang, L., Donfield, S., Goedert, J. J., Phair, J., … Vlahov, D. (2002). Modulating influence on HIV/AIDS by interacting RANTES gene variants. Proceedings of the National Academy of Sciences of the United States of America, 99(15), 10002–10007. Web.
Apostolova, N., Funes, H. A., Blas-Garcia, A., Galindo, M. J., Alvarez, A., & Esplugues, J. V. (2015). Efavirenz and the CNS: What we already know and questions that need to be answered. Journal Antimicrob. Chemother, 70(1), 2693–708. Web.
Boliar, S., Gludish, D. W., Jambo, K. C., Kamng’ona, R., Mvaya, L., Mwandumba, H. C., … Russell, D. G. (2018). Inhibition of the lncRNA SAF drives activation of apoptotic effector caspases in HIV-1–infected human macrophages. Proceedings of the National Academy of Sciences of the United States of America, 116(13), 1-21.
Cagigi, A., Du, L., Dang, L. V., Grutzmeier, S., Atlas, A., Chiodi, F., … Nilsson, A. (2009). CD27(-) B-cells produce class switched and somatically hyper-mutated antibodies during chronic HIV-1 infection. PloS one, 4(5), 1-27. Web.
Chan, D. P., Lee, M., Wong, N., Leung, R. K., Naftalin, C. M., … Lee, S. (2016). Association of immune recovery with hyperlipidaemia and apolipoprotein gene polymorphisms following highly active antiretroviral therapy in a cohort of Chinese HIV patients. BMJ Open, 6(4), 1-7. Web.
Chevalier, M. F., & Weiss, L. (2013). The split personality of regulatory T cells in HIV infection. Blood, 121(1), 29-37.
Conway, J. M., Konrad, B. P., & Coombs, D. (2013). Stochastic analysis of pre- and postexposure prophylaxis against HIV infection. SIAM Journal on Applied Mathematics, 73(1), 904-928. Web.
d’Ettorre, G., Baroncelli, S., Micci, L., Ceccarelli, G., Andreotti, M., Sharma, P., … Fanello, G. (2014). Reconstitution of intestinal CD4 and Th17 T cells in antiretroviral therapy suppressed HIV-infected subjects: Implication for residual immune activation from the results of a clinical trial. PLoS One, 9(10), 1-9. Web.
Dellar, R. C., Dlamini, S., & Karim, Q. A. (2015). Adolescent girls and young women: Key populations for HIV epidemic control. Journal of the International AIDS Society, 18(2), 21-33. Web.
Dickinson, L., Amin, J., Else, L., Boffito, M., Egan, D., Owen, A., … Khoo, S. (2015). Pharmacokinetic and pharmacodynamic comparison of once-daily efavirenz (400 mg vs. 600 mg) in treatment-naïve HIV-infected patients: results of the ENCORE1 study. Clinical Pharmacology & Therapeutics, 98(1), 406-416. Web.
Dickinson, L., Amin, J., Else, L., Boffito, M., Egan, D., Owen, A., … Back, D. (2016). Comprehensive pharmacokinetic, pharmacodynamic and pharmacogenetic evaluation of once-daily efavirenz 400 and 600 mg in treatment-naïve HIV-infected patients at 96 weeks: Results of the encore1 study. Clinical Pharmacology & Therapeutics, 55(3), 861-873. Web.
Duwal, S., & von Kleist, M. (2016). Top-down and bottom-up modeling in system pharmacology to understand clinical efficacy: an example with NRTIs of HIV-1. European Journal of Pharmaceutical Sciences, 94(1), 72-83. Web.
Duwal, S., Dickinson, L., Khoo, S., & von Kleist, M. (2018). Hybrid stochastic framework predicts efficacy of prophylaxis against HIV: An example with different dolutegravir prophylaxis schemes. PLOS Computational Biology, 14(2), 1-18. Web.
Duwal, S., Dickinson, L., Khoo, S., & von Kleist, M. (2019). Mechanistic framework predicts drug-class specific utility of antiretrovirals for HIV prophylaxis. PLOS Computational Biology, 15(40), 1-18. Web.
Duwal, S., Seeler, D., Dickinson, L., Khoo, S., & von Kleist, M. (2019). The utility of efavirenz-based prophylaxis against HIV infection: A systems pharmacological analysis. Frontiers Pharmacology, 2(11), 4-19.
Duwal, S., Sunkara, V., and von Kleist, M. (2016). Multiscale systems-pharmacology pipeline to assess the prophylactic efficacy of NRTIs against HIV-1. CPT: Pharmacometrics & Systems Pharmacology, 5(1), 377-387. Web.
Fletcher, C. V., Staskus, K., Wietgrefe, S. W., Rothenberger, M., Reilly, C., Chipman, J. G., … Beilman, G. J. (2014). Persistent HIV-1 replication is associated with lower antiretroviral drug concentrations in lymphatic tissues. PNAS, 111(2), 2307-2312. Web.
Grant, R. M., Anderson, P. L., McMahan, V., Liu, A., Amico, K. R., Mehrotra, M., … Hosek.S. (2014). Uptake of pre-exposure prophylaxis, sexual practices, HIV incidence in men transgender women who have sex with men: a cohort study. The Lancet Infectious Diseases,14(2), 820-829. Web.
Gutiérrez, C., Lopez-Abente, J., Pérez-Fernández, V., Prieto-Sánchez, A., Correa-Rocha, R., Moreno-Guillen, S., Muñoz-Fernández, M. Á. (2019). Analysis of the dysregulation between regulatory B and T cells (Breg and Treg) in human immunodeficiency virus (HIV)-infected patients. PLoS One, 14(3), 1-16. Web.
He, L., Pan, X., Dou, Z., Huang, P., Zhou, X., Peng, Z., … Zheng, J. (2016). The factors related to CD4+ T-cell recovery and viral suppression in patients who have low CD4+ T cell counts at the initiation of HAART: A retrospective study of the national HIV treatment sub-database of Zhejiang province, China, 2014. PLoS One. 11(2), 1-14. Web.
Jespersen, S., Tolstrup, M., Hønge, B. L., Medina, C., da Silva, D. T., Ellermann-Eriksen, S., … Østergaard, L. (2015). High level of HIV-1 drug resistance among patients with HIV-1 and HIV-1/2 dual infections in Guinea-Bissau. Virology Journal, 12(41), 1-6. Web.
Lee, B., Doranz, B. J., Rana, S., Yi, Y., Mellado, M., Frade, J. M., … Doms, R. W. (1998). Influence of the CCR2-V64I polymorphism on human immunodeficiency virus type 1 coreceptor activity and on chemokine receptor function of CCR2b, CCR3, CCR5, and CXCR4. Journal of virology, 72(9), 7450–7458.
Lee, S. S., Wong, N. S., Wong, B. C., Wong, K. H., & Chan, K. C. (2017). Combining CD4 recovery and CD4: CD8 ratio restoration as an indicator for evaluating the outcome of continued antiretroviral therapy: an observational cohort study. BMJ Open, 7(9), 1-12. Web.
Liu, J., Zhan, W., Kim, C. J., Clayton, K., Zhao, H., Lee, E., … Ostrowski, M. (2014). IL-10-producing B cells are induced early in HIV-1 infection and suppress HIV-1-specific T cell responses. PloS One, 9(2), 1-22. Web.
Mahajan, S. D., Agosto-Mojica, A., Aalinkeel, R., Reynolds, J. L., Nair, B. B., Sykes, D. E., … Martinez, J. (2010). Role of chemokine and cytokine polymorphisms in the progression of HIV-1 disease. Biochemical and Biophysical Research Communication, 396(2), 348-52. Web.
Merci, N. M., Emerence, U., Augustin, N., Habtu, M., Julie, I., Angelique, T., … Jessica, B. (2017). CD4+ cells recovery in HIV positive patients with severe immunosuppression at HAART initiation at center medico-social Cor-Unum, Kigali. Pan African Medical Journal, 26(14), 1-13. Web.
Moir, S., Ho, J., Malaspina, A., Wang, W., DiPoto, A. C., O’Shea, M. A., … Roby, G. (2008). Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. Journal of Experimental Medicine, 205(8), 1797-805. Web.
Nakanjako, D., Ssewanyana, I., Mayanja-Kizza, H., Kiragga, A., Colebunders, R., Manabe, Y. C., … Nabatanzi, R. (2011). High T-cell immune activation and immune exhaustion among individuals with suboptimal CD4 recovery after 4 years of antiretroviral therapy in an African cohort. BMC Journal, 11(43), 1-11. Web.
Nikolova, M., Wiedemann, A., Muhtarova, M., Achkova, D., Lacabaratz, C., … Lévy, Y. (2016). Subset- and antigen-specific effects of Treg on CD8+ T cell responses in chronic HIV infection. PloS One, 12(11), 1-22.
Onywera, H., Maman, D., Inzaule, S., Auma, E., Were, K., Fredrick, H., … Owiti, P. (2017). Surveillance of HIV-1 pol transmitted drug resistance in acutely and recently infected antiretroviral drug-naïve persons in rural western Kenya. PLoS One, 12(2), 1-14. Web.
Philpott, S., Burger, H., Tarwater, P. M., Lu, M., Gange, S. J., Anastos, K., … Weiser, B. (2004). CCR2 genotype and disease progression in a treated population of HIV type 1-infected women. Clinical infectious diseases: An official publication of the Infectious Diseases Society of America, 39(6), 861–865. Web.
Ponnan, M. S., Pattabiram, S., Thiruvengadam, K., Goyal, R., Singla, N., Mukherjee, J., … Chatrath, S. (2019). Induction and maintenance of bi-functional (IFN-γ + IL-2+ and IL-2+ TNF-α+) T cell responses by DNA prime MVA boosted subtype C prophylactic vaccine tested in a Phase I trial in India. PLoS ONE 14(3), 1-16.
Ponnan, M. S., Swaminathan, S., Tiruvengadam, K., Vidyavijayan K. K., Cheedarla, N., Nesakumar, M., … Kathirvel, S. (2018). Induction of circulating T follicular helper cells and regulatory T cells correlating with HIV-1 gp120 variable loop antibodies by a subtype C prophylactic vaccine tested in a Phase I trial in India. PLoS One, 13(9), 1-11.
Restrepo, C., Gutierrez-Rivas, M., Pacheco, Y. M., García, M., Blanco, J., Medrano, L. M., … Navarrete-Muñoz, M. A. (2019). Genetic variation in CCR2 and CXCL12 genes impacts on CD4 restoration in patients initiating CART with advanced immunesupression. PLoS One, 14(3), 1-13. Web.
Rhee, S., Blanco, J. L., Jordan, M. R., Taylor, J., Lemey, P,, Varghese, V., … Hamers, R. L. (2015). Geographic and temporal trends in the molecular epidemiology and genetic mechanisms of transmitted hiv-1 drug resistance: An individual-patient- and sequence-level meta-analysis. PLoS One, 12(4), 1-29. Web.
Siewe, B., Wallace, J., Rygielski, S., Stapleton, J. T., Martin, J., Deeks, S. G., & Landay, A. (2014). Regulatory B cells inhibit cytotoxic T lymphocyte (CTL) activity and elimination of infected CD4 T cells after in vitro reactivation of HIV latent reservoirs. PloS one, 9(4), 1-15. Web.
Soria, A., Guerini, F. R., Bandera, A., Bolognesi, E., Uglietti, A., Fusco, C., … Zucchi, P. (2011). KIR-HLA genotypes in HIV-infected patients lacking immunological recovery despite effective antiretroviral therapy. PLoS One, 1(14), 3-8.
Tang, M. W., & Shafer, R. W. (2012). HIV-1 antiretroviral resistance: Scientific principles and clinical applications. Drugs, 72(9), 1-25. Web.
Trifone, C., Salido, J., Ruiz, M. J., Leng, L., Quiroga, M. F., Salomón, H., … Turk, G. (2018). Interaction between macrophage migration inhibitory factor and CD74 in human immunodeficiency virus type i infected primary monocyte-derived macrophages triggers the production of proinflammatory mediators and enhances infection of unactivated CD4+ T cells. Frontiers in immunology, 9(1494), 11-23. Web.
Zoufaly, A., Cozzi-Lepri, A., Reekie, J., Kirk, O., Lundgren, J., Reiss, P., … Jevtovic, D. (2014). Immuno-virological discordance and the risk of non-AIDS and AIDS events in a large observational cohort of HIV-patients in Europe. PLoS One, 9(1), 1-15. Web.