Introduction
Piezoelectric nano-biosensors are designed to operate in the nano-dimensions. They are mainly used to conduct an analysis of various biomaterial samples with the chief objective of understanding bio-compositions, functionalities, and even their structures (Atta 2011).
Although different modes of operations are deployed by different biosensors, their central principle of operation is based on the interactions of physical and or chemical detectors and the biological components. Through this interaction, it becomes possible to detect and analyse analytes (Joshi & Bhansali 2008).
A biological recognition element that incorporates a nano-material or nano-scale phenomena having the capability to convert the cognized event through transduction process into an appropriate signal in chemical of physical format is the building block of piezoelectric nano-biosensors.
In these sense, piezoelectric nano-biosensors form the bridge between the biological spectrum and the chemical and or physical spectrums (Khana 2012).
Some of the detection principles of operation of nano-biosensors include optical, mechanical, chemical, and piezoelectric detection ideologies. The focus of this paper is on the piezoelectric bio-sensing detection principle.
The science behind piezoelectric nano-biosensors
Piezoelectric transducers are deployed in the signal detection process to change mechanical force that is exacted by masses placed on a crystal onto some electrical signal, which while amplified is used to proportionately measure the mass of an object placed on the piezoelectric material.
When a mass is placed on top of piezoelectric material, the material resonates with a frequency proportional to the mass placed on it. A voltage proportionate to the size of the mass is generated (Dey & Goswami 2011).
On the nano-dimensions, piezoelectric materials or nano-biosensors deploying the piezzo electric principle of operation possess immobilised elements of sensing on some piezoelectric crystals.
A good example of material that can be deployed to function as a piezoelectric transducer is “functionalised quartz crystal with dehydrogenate to detect formaldehyde” (Pohanka et al. 2007: 2826).
However, it is perhaps also crucial to note that, when voltage is applied across a piezoelectric material, the material deforms proportionately to the amount of voltage applied.
An immense theoretical study on the operation principle of the piezoelectric material has been developed. Whether at nano level or even in the kilo level, the basic principle of operation remains the same.
Sauerbrey (Ho 1999), Stockridge (Hahn 1988) and Lostis (Muramatsu et al. 1987) deployed different approaches to develop equations that describe the relationship between the frequency of resonance for an oscillating crystal of a piezoelectric material and a mass placed on the surface of the crystal.
Although the three scholars’ equations are similar, Sauerbrey’s equation has received a universal acceptance (Ho 1999).
Sauerbrey’s equation relates to an AT-cut quartz crystal “vibrating in the thickness shear mode that describes the relationship between mass of thin films deposited on the quartz crystal and the corresponding change in resonant frequency of the crystal” (Hussain et al. 1997: 505).
The change in frequency for an oscillating quartz crystal in Hz is given by the equation:
Source: (Hussain et al. 1997: 505)
In the equation,
denotes the change in mass that produces a corresponding variation in frequency
. A is the electrode surface area measured in cm2. The described relationship by Sauerbrey is not only applicable to film depositions but equally applicable to deposition of particulate matter (Hussain et al. 1997: 505).
In the thickness-shear mode of vibration, the overall frequency of oscillation of AT-cut quartz crystal is described by the equation:
F defines the crystal frequency, N is a constant of the material used to make the piezoelectric sensor, and ‘a’ defines the thickness of the material used to make the crystal.
For a quartz crystal, which is the most typical material deployed to make piezoelectric transducers, N is given by 1.66 MHz-mm. The thickness of a crystal can be defined by the equation:
Where M stands for the electrically driven portion of the mass of the crystal, A the eclectically driven crystal area, and Rho (⍴) defines the crystal’s density.
Since the crystal of quartz is in solid state, its density is constant. Consequently, for an infinite change in mass M, the corresponding change in frequency registered by the piezoelectric transducer is given by:
Source: (Hussain et al. 1997: 505)
However, in the practical application of piezoelectric transducer, F and M are constants so that
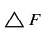
is given by
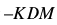
where K is the constant defined by
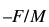
This equation shows that the change in frequency produced by a piezoelectric biosensors inserted in an analyte is proportionate to the change in mass. This principle can be applied in the construction of piezoelectric biosensor used in treatment of tuberculosis process as described later in the paper.
Example of a nano-biosensor, which utilises piezoelectric detection principle
A good example of biosensor that utilises piezoelectric principle is the micro membrane biosensors. The sensor makes use of thin films of crystal falling in the range of 10 to 30nm (Woolley, 2000). When placed near cells, they are able to detect the masses of the cells and hence their presence.
The detection process is realised via measurements of natural frequencies of the vibrating crystals. The frequency is a function of the mass placed on the piezoelectric sensing element.
Applying such sensors in the immunology demands that the masses of cell belonging to infectious organizations are known so that the frequency registered can be used to identify the type of pathogen in the body of a host.
In some situations, during infections, the pathogen cell interacts with body defence mechanism cells, which comprise the antibodies through the engulfing process.
When such a process occurs, rapid changes in the frequencies of the piezoelectric nano-sensor occur so that, even if the immunologist may not be fully cognizant about the specific type of infection a patient is suffering from, he or she can be able to detect anomalies in the patient’s body.
When micro-membrane biosensors are used this way, they form an essential component of pre-diagnosis process.
Difference of nano biosensors and conventional non-nano methods: advantages and disadvantages
Interaction of antibodies with antigens provides a wonderful opportunity for development of chemical binding based biosensor.
In theoretical terms, in case it is possible to raise an antibody against a specific analyte, it becomes possible to develop an immune-sensor to recognise it, for which piezoelectric sensor is one of the imuno-sensors.
It is also important to note, “Despite high specificity and affinity of antibodies towards complementary ligand molecules, most antibody-antigen interactions do not cause an electronically measurable change” (Kumar 2008: 198).
This forms a major challenge of application of nano biosensors in the detection of changes in the analyte masses for possible detection of pathogens, for instance, those causing TB.
Nonetheless, this argument does not mean that it is impossible to develop a piezoelectric biosensor such as the one described in the subsequent sections of the paper.
Piezoelectric biosensors can be produced from a myriad of crystalline substances. Such substances have incredible properties, which facilitates precise detection of analytes for presence of antigen-antibody reactions.
Indeed, a piezoelectric “immune-sensor is thought to be one of the most sensitive analytical instruments developed to date, being capable of detecting antigens in the pictogram range” (Kumar 2000: 198).
Opposed to traditional sensors, the piezoelectric biosensors can detect the antigens while in both liquid and gaseous phases.
When the developed device is applied in detection of changes in mass of amalgamations formed due to reaction between the antigens and antibodies because of infection of tuberculosis bacterium, principle advantages are gained while the functionality of the device is compared with the traditional approaches in tuberculosis detection procedures and processes.
In fact, the traditional approaches to diagnosis of tuberculosis have repetitive drawbacks. Generally, they are time consuming. In some instances, they are non-specific.
Kumar (2000) exemplifies this argument by asserting, “In most cases of pulmonary and extra-pulmonary TB, diagnosis depends upon culturing the micro-bacterial organism, a process requiring 4-8 weeks” (199).
Consequently, in the attempt to counter this challenges, magnificent attention has been directed towards development of mechanisms of diagnosis of TB, which are rapid and time cautious by various researchers.
Some of the developed strategies fail to have high sensitivity and specificity for the required appropriate diagnosis (Kumar 2000). It is for this reason that the proposed piezoelectric biosensor is both appropriate and significant in driving new approaches for tuberculosis detection or diagnosis.
The science and principles of operation of traditional biosensors do not constitute an adequate basis for advocating their utilisation in practice. Issues such as economic factors in relation to their manufacturing coupled with their functionalities are also critical.
The main question is, ‘how effective are the traditional biosensors in realisation of the purposes they are designed to accomplish?’
The rapid explosions of technology and science experienced over the last three decades pose challenges to the applicability of the traditional biosensors in the era of scientific and technological evolution.
Such challenges manifest themselves in terms of improvement of the accuracy and preciseness of the transducers, multiplexing (the capacity for detection of biomarkers in a simultaneous manner), reduction of costs associated with manufacturing, and operation of the gadgets (Prakash et al 2012: Bhushan 2012).
It is through the need to resolve some of these challenges of the traditional biosensors that the nano-biosensors have been developed.
A particular concern for embracement of the proposed piezoelectric nano-biosensors in the diagnosis of TB is articulated to the need to increase the specificity and sensitivity of the traditional biosensors in the quest to facilitate early detection of the associated signals to be measured.
A major advantage of using the nano-biosensors in opposition to the traditional biosensors is that, while traditional biosensors utilise “any specific substance “as the analyte, the nano-biosensor uses “any substances with the potential to detect a single molecule or fragment of DNA” (Adam & Kizek 2008, p.6129).
This argument means that the detection is narrowed to molecular level. Nano-bio-sensing is achieved in the same manner under various approaches.
These approaches include the use of antigen-antibody interactions, cell based, and enzymes catalysis and through nucleic acid recognition. Nonetheless, nano-bio-sensing extends this approach to include ELISA and nanonfluidic technology and techniques.
Mass-production of the sensor, details of the fabrication method and cost-effectiveness
Mass production
In the mass production of products, reproducibility is an important factor. In mass production of piezoelectric nano-biosensors, reproducibility is a major hindrance. The sensitivity and precision of piezoelectric biosensor are directly proportional to the dimensional accuracy including the shape of the crystal cut.
The obstacles coupled with other obstacles such as “presence of biomaterial in the biosensor (immobilisation of bimolecules on transducers, stability of enzymes and antibodies), the development of the sensor device (sensitivity and reproducibility issues) and the integration of biosensors into complete systems” (Velasco-Garcia & Mottram 2003: 5) impair the mass production of the piezoelectric biosensor.
However, with new advents of production technologies such as rapid prototyping and computer aided precision machining, it is possible to mass-produce the device’s components.
Details of the fabrication
In this section, a practical piezoelectric biosensor is described. Since a nano-piezoelectric biosensor would require highly precise machining process and intricate fabrication techniques, which are not realisable in the context of this paper, the discussed example of piezoelectric biosensor does not fall in the nano range.
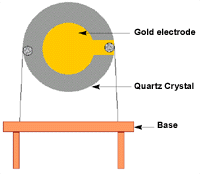
The block diagram (Fig 1) below illustrates the schematically the components of the nano-biosensor showing how they are related with one another.

Fig 1: Block diagram for the piezoelectric sensor
Source: (Pohanka et al. 2007)
The piezoelectric sensor is composed of three main components. These are gold electrode, quartz crystal, and the base plate. Quartz crystal used is made of alpha quartz since such a crystal is not soluble in water. It is also resistant to high temperatures (Pohanka et al. 2007).
Indeed, alpha quartz crystal has the capacity to withstand temperature up to 579 degree centigrade without losing prosperities of piezoelectric. The resonance frequency of the quartz crystal is a function of the physical dimensions and measurements of the cut crystal.
AT-cut crystal is the most preferred for the discussed piezoelectric biosensor since it has been proved through scholarly researches that it is the most stable. Its temperature coefficient is 1ppm per degree centigrade within a temperature range of 10 degrees centigrade to 50 degree centigrade.
This property is important since the temperature under which the piezoelectric sensor proposed here will operate (under environmental standard temperature) is in this temperature range.
The crystal can also be modified to take a number of forms including rectangles, discs, and squares. The figure below shows schematically the details of the fabrication of the sensor as discussed by Kumar (2000).
Source: (Kumar 2000)
In the process of imuno-sensing, a protein extracted from Staphylococcus aureus is used to modify the surface of the coating of the device to increase the adhesion properties of the antibodies. The form of Staphylococcus protein used is capable to bind on the molecules of the imunoglobin particularly the IgG antibodies.
When immersed in a tube containing antigens, it is anticipated that antigen-antibody reaction will occur thus causing a change in the mass on the crystal. From the developed theory of operation of the device, a corresponding frequency change occurs.
The frequency counter will record this change in frequency as a change (△F). Since antibody-antigen reaction is anticipated to occur in magnitudes corresponding to the concentration levels of antigens, the higher the frequency changes, the higher the concentration of antigens.
Consequently, it is more likely for the patients from whom the antigens have been obtained to suffer from advanced TB.
Cost effectiveness
One of the central challenges on the commercial production of nano-sensors is associated with the cost of production of micro components of the sensors with high degrees of precision.
Although, over the last two decades, these costs have been significantly high, the developments in the nanotechnology has made is possible to produce components for biosensors at significantly low costs (Joshi, Sharma & Harsha, 2011).
The rapid developments in production process such as rapid prototyping make it possible for the sensor to be produced in a cost effective manner.
Conclusion
Piezo nano-biosensors encompass the nano-scale sensing devices deployed to detect a myriad of analytes including microorganisms, proteins, nucleic acids, metal, and metabolites among others. In the production of these sensors, nanotechnology is playing pivotal role.
In this context, the paper argues that nanotechnology has resulted in the revolution of the immunology technology in the extent that it is now possible to apply biosensors to detect presence of pathogens at the molecular level. This provides a possibility for early treatment.
By fully appreciating that there are many detection principles that can be applied in the development of nano-biosensors, the paper limited itself to the piezoelectric principle of detection. A possible piezoelectric sensor has been discussed in the paper alongside with its fabrications and operation principles.
The paper argued that deploying antigens-antibody mechanism of detection of increases in masses resulting from antibody- antigens reactions due to the presence of Staphylococcus aureus. The sensor can aid in the early diagnosis of tuberculosis than it would ordinarily happen by the use of convectional nano-biosensors.
Bibliography
Adam, V & R Kizek, ‘Utilization of Electrochemical Sensors and Biosensors in Biochemistry and Molecular Biology’, Sensors, vol. 8, no. 10, 2008, pp.6125–6131.
Atta, N, A Galal, & S Ali, Nanobiosensors for health care, Biosensors for Health, Environment and Biosecurity, 2011. Web.
Bhushan, B, ‘Biosensors: surface structures and materials’, Philosophical transactions, Mathematical, physical, and engineering sciences, vol. 370, no.1967, 2012, pp.2267–2268.
Dey, D & T Goswami, ‘Optical Biosensors: A Revolution towards Quantum Nanoscale Electronics Device Fabrication’, Journal of Biomedicine and Biotechnology, vol.12, no. 5, 2011, pp 356-361.
Hahn, C Piezoelectric Crystal Detectors and Their Applications, University of New Orleans, New Orleans, 1998.
Ho, M, Applications of Piezoelectric Quartz Crystal Microbalances, Amsterdam, Elsevier, 1999.
Hussain, I et al. ‘Fabrication of Piezoelectric Sensors for Biomedical Applications’, MRS Symp. Proc. Materials for Smart System, vol. 459, no.31, 1997, pp. 501-506.
Joshi, Y, C Sharma, & P Harsha, ‘Zeptrogram scale mass sensing using single walled carbon nanotube based biosensors’, Sensors and Actuators A: Physical, vol. 168, no,11, 2011, pp. 275-280.
Joshi, R & S Bhansali, ‘Nanosensor Technology’, Journal of Nanomaterials, vol. 1, no. 1, 2008, pp. 1-10.
Khana, V, Nanosensors: Physical Chemical and Biological, Florida: CRC Press.
Kumar, K 2000, ‘Biosensors Based on Piezoelectric Crystal Detectors: Theory and Application’, JOM, vol. 52, no.10, 2012, pp. 198-207.
Muramatsu, H et al. ‘Piezoelectric Crystal Biosensor Modified with Protein A for Determination of Immunoglobulins’, Analytical Chemistry, vol. 59, no.19, 1987, pp. 2760-2763.
Pohanka, M et al. ‘Piezoelectric Biosensor for a Simple Serological Diagnosis of Tularemia in Infected European Brown Hares’, Sensors, vol.7, no.11, 2007, pp. 2825–2834.
Prakash, S, M Pinti, & B Bhushan, ‘Theory, fabrication and applications of microfluidic and nanofluidic biosensors’, Philosophical transactions, Mathematical, physical, and engineering sciences, vol. 370, no.196, 2012, pp. 2269–2303.
Velasco-Garcia, M & T Mottram, ‘Biosensor Technology addressing Agricultural Problems’, Biosystems Engineering, vol. 84, no.1, 2003, pp. 1–12.
Woolley, T, L Cheung, H Hafner, & M Lieber, ‘Structural biology with carbon nanotube AFM probes’, Chemistry and Biology, vol. 7 no. 3, 2000, pp. 192-204.