Synthesis of Plasmonic Gold Nanoparticles
- How does the solution visibly change? Record your observations in the table 1 (5 points)
Table 1.
- Why do you think during the reaction some intermediate colors are seen? (10 points)
Characterization and Property of the Synthesized Plasmonic Gold Nanoparticles
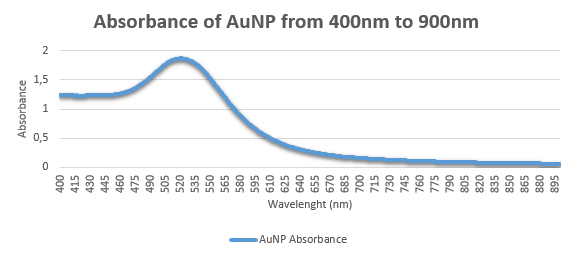
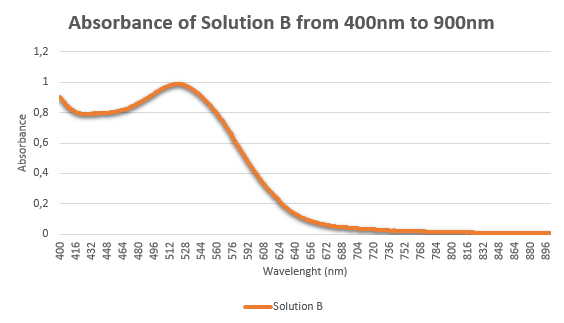
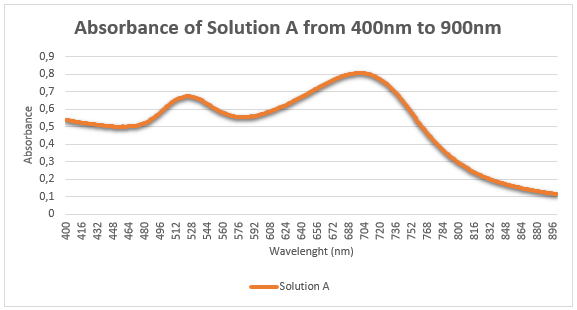
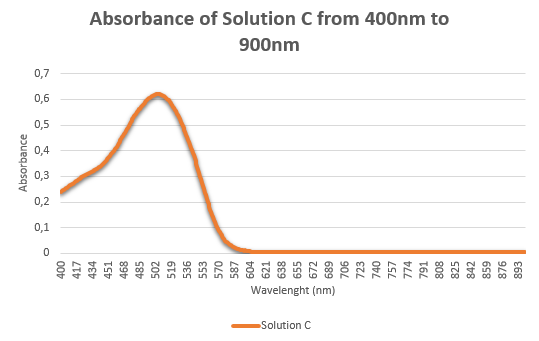
- You are provided with 3 ruby-red samples containing: red wine, food colorant, and AuNP solution. These three samples are labelled as A, B, and C. Based on the Tyndall light scattering effect, salt-induced aggregation LSPR properties, which sample contain AuNPs? Present the evidences that help you identify the AuNP solution. Please explain in detail (25 points)
- You are provided with three colourless samples containing: casein, sugar, and salt. These samples are labelled as A, B, and C. Using the negative charged citrate-stabilized AuNPs that you have synthesized, identify what the sample A, B, or C contains about. Present the evidences that help you identify the samples A, B, and C. Please explain in detail (25 points)
Development of Colorimetric Nanosensor for Detection of Ascorbic Acid (Vitamin C)
Record the measured absorbance values into Table 3 (10 points)
Table 3.
- What is the estimated concentration of the blind sample based on the colour tonality of the diluted concentrations? Present with a colour photograph taken by your group. (5 points) 1.8mM
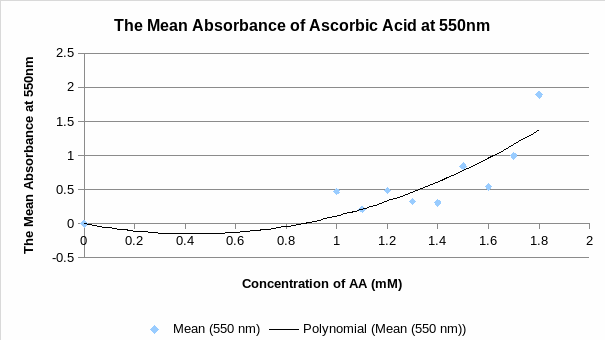
- Plot a calibration curve between mean values A550 nm and AA concentrations that you recorded in Table 3. The value of STDEV (standard deviation) should be presented in the curve. Is the calibration curve linear within this concentration range? (10 points)
The calibration curve does not appear linear within the range of concentration of 0 to 2mM because logarithmic scale fit the data points more than a linear scale.
- A linear relationship between mean values A550 nm and AA concentrations could be obtained if your results are good enough. Plot your linear fitted curve. Based on the linear regression, what is the detection limit of the assay? (5 points); and what is the AA concentration of the blind sample? (5 points)
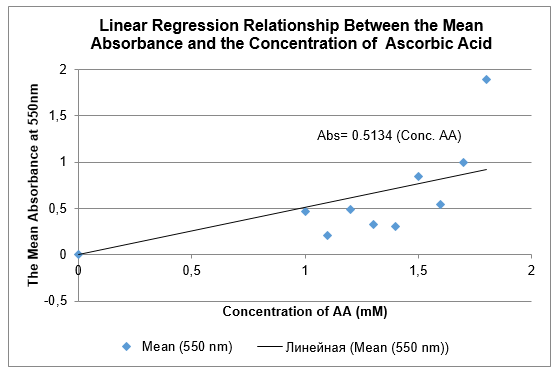
Absorbance = 0.5134(Conc. AA)The detection limit of AA, which is triple the standard deviation of the blank solution with background noices (Workman 2016), is 0.003 (0.001*3).
Unknown sample absorbance = 1.918
Therefore, the concentration of the unknown sample of AA = 1.918/0.5134 = 3.736nM
References
Stoker, S 2015, General, organic, and biological chemistry, Cengage Learning, Mason, OH.
Tyagi, H, Kushwaha, A, Kumar, A, & Aslam, M 2016, ‘A facile pH controlled citrate-based reduction method for gold nanoparticle synthesis at room temperature’, Nanoscale Research Letters, vol. 11, no. 1, pp. 1-11.
Workman, J 2016, The concise handbook of analytical spectroscopy: theory, applications, and reference materials, World Scientific, New Jersey, NJ.