Introduction
One of the main tasks of modern engineering sciences is to find alternative materials that demonstrate high value for human industry, including medicine. Traditionally found solutions have a range of physical and chemical properties useful for manufacturing: material transparency, semi-permeability, chemical activity, flexibility, strength, or fluidity. In other words, the materials chosen for the solution of applied problems have several advantages at once, which makes them useful.
Poly(methyl acrylate), PMA, has long established itself as a successful raw material for the industrial production of acrylic acid polymers and as a finishing agent for leather and textiles, but in recent decades, the role of this compound in medicine was actively growing. This product’s range of action extends from its application as a biocompatible additive to eliminate the undesirable effects of metal medical instruments to its use as a copolymer for targeted drug delivery. The purpose of this work is to discuss this material in detail and identify the most promising medical uses.
The Material
The exceptional narrowness of poly(methyl acrylate) use in industry is closely linked to the unique characteristics of the material in comparison with other polymers of acrylic acid. Depending on the production method, PMA can be quite soft, flexible semitransparent sheets of dense plastic. Regardless of the synthesis scheme, PMA retains hydrophobicity and complex surface morphology due to its chemical nature (Figure 1). From a chemical point of view, the polymer is obtained by catalytic reaction of free-radical polymerization of methacrylic or acrylic acids, or their derivatives, including esters, as shown in Equation 1.
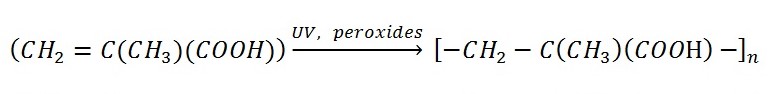
Equation 1. Schematic process of polymerization reaction under the influence of UV and catalysts. The degree of polymerization of the finished product is from several hundred to several thousand.
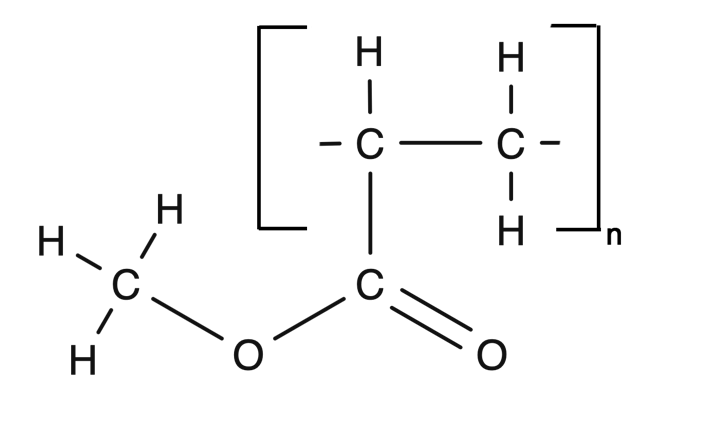
Commercial poly(methyl acrylate), (C4H6O2)n, is traditionally supplied as powder suspensions with a fixed granule size. Although the product has a relatively high self-ignition temperature (896 °F), its storage requires special conditions since, above 86 °F, the suspension tends to form conglomerates (“Poly(methyl acrylate),” n.d.). The polymer’s physical and chemical properties have led to its high applicability as solvents, which is also reflected in the pharmaceutical industry. Thus, PMA is registered in the FDA database as an inactive component of medicines for oral administration.
Medical Uses
The fields of application of poly(methyl acrylate) in clinical practice are not limited to a few narrow examples. On the contrary, the specific solubility properties of organic substances, hydrophobicity, and elasticity of the polymer stipulate a wide range of practical uses. Thus, the most significant areas are the use of poly(methyl acrylate) as a means to create a film-coating structure of drug capsules, the cover of metal medical instruments to increase biocompatibility with soft tissues of the body, and the use of the substance as a copolymer for targeted drug delivery.
Of primary importance is the recognition that the use of PMA in clinical practice is justified by the substance’s harmlessness to the human body. Studies of drug toxicity have shown that daily consumption of up to 200 mg per kilogram of body weight is almost safe for humans (“Poly(methyl acrylate),” n.d.). Given this fact, pharmacists widely use modified versions of the polymer as a film-coating formation substance for capsule drugs. The main trademarks of the drug are Eudragits, specific types of which are aimed at different acidity of the medium. For example, Eudragit E is a substance soluble under the action of gastric juice, while Eudragit L is an enteric coating agent; in other words, resistant to the pH of the stomach.
On the different hand, the drug has found wide use as a micelle co-material for targeted drug delivery. It should be recognized that the central problem of this field of medicine is to find the ideal nanocomposite capable of targeting the substrate and not harming the body. Grace et al. (2017) showed that the use of modified poly(methyl acrylate) in the synthesis of lipid polymers is of high value since it allows for stabilizing the liquid crystal nanoparticle and preserving the internal structure of the micelle filled with the drug.
Meanwhile, an interesting effect of the combination of PMA/PMMA polymers is to improve the distribution of the active ingredient delivered to the colon tissue of laboratory rats (Youshia & Lamprecht, 2016). These results suggest promising opportunities for the use of poly(methyl acrylate) as a sheath component for spot binding to the substrate. Simultaneously, Xia et al. (2016) argued that aromatic organic molecules, such as spirogyra, integrated into PMA showed high sensitivity to ultrasound through the destruction of chemical bonds and restructuring of the atomic grouping. The researchers expressed hope that such properties will form the basis of self-healing materials that counteract the destruction of human tissues.
Poly(methyl acrylate) plays an equally important role as a biopolymer coating for medical instruments. It is known that interacting with soft tissues of the skin, organs, and mucous membranes, tweezers, scalpels, and blades made of steel or heavy metals can cause local irritation. Preventing the undesirable effects of treatment is a fundamental problem in clinical practice, and the use of poly(methyl acrylate) can partially solve it. In particular, the polymer, in combination with acrylic acid, shows high biocompatibility with liquid structures of the body, including blood (Yahata, 2017). This means that the application of copolymer mixture on the instruments will inhibit the release of heavy metal ions into the blood, which obviously increases the effectiveness of surgical practice.
References
Grace, J. L., Alcaraz, N., Truong, N. P., Davis, T. P., Boyd, B. J., Quinn, J. F., & Whittaker, M. R. (2017). Lipidated polymers for the stabilization of cubosomes: nanostructured drug delivery vehicles. Chemical Communications, 53(76), 10552-10555. Web.
Poly(methyl acrylate). (n.d.). Chemical Book. 2020. Web.
Xia, H., Zhao, Y., & Tong, R. (2016). Ultrasound-mediated polymeric micelle drug delivery. In J.- M. Escoffre & A. Bouakaz (Eds.), Therapeutic ultrasound (pp. 365-384). Springer.
Yahata, C., Suzuki, J., & Mochizuki, A. (2019). Biocompatibility and adhesive strength properties of poly (methyl acrylate-co-acrylic acid) as a function of acrylic acid content. Journal of Bioactive and Compatible Polymers, 34(6), 479-493. Web.
Youshia, J., & Lamprecht, A. (2016). Size-dependent nanoparticulate drug delivery in inflammatory bowel diseases. Expert Opinion on Drug Delivery, 13(2), 281-294.