Brief Statement of Purpose of Experiment
The central purpose of this experiment is to study the primary salt effect in the oxidation reaction of iodide ions to iodine during sodium thiosulfate titration with appearance of salt KNO3
Procedure Reference
All steps of the procedure of this experiment were performed according to the theoretical guidelines given by Amdur & Hammes (1966), Knudsen & King (1938), and Indelli & Prue (1959). Initially, three working solutions and one solvent had to be prepared using distilled water: 250 mL of 0.1 M potassium iodide, 150 mL of 0.001 M sodium thiosulfate, and 300 mL of 0.01 M potassium persulfate. The solvent substance contained 0.001 N hydrogen chloride and 0.00001 M EDTA. Then, 200 mL of 1 M potassium nitrate solution was obtained with the solvent. A mixture of 1 g of starch in 100 mL of water was prepared as an indicator by gradual heating until homogeneous. A series of ten Erlenmeyer flasks were used to obtain different amounts of different prepared solutions according to the table below. The time range of each reaction which was counted from the first drop of potassium persulfate interacting with the solution in the flask until the blue color appeared was considered as ∆t.
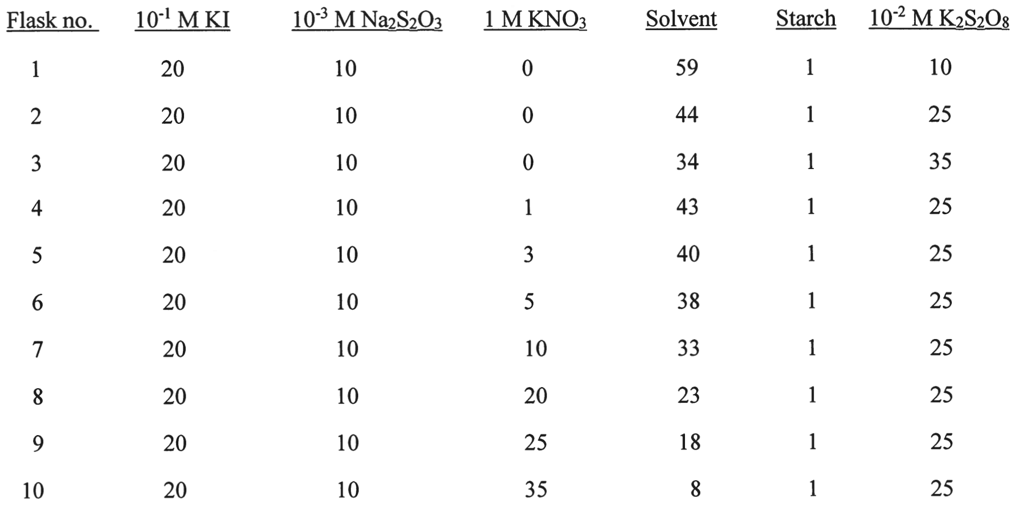
All Chemical Reactions
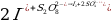
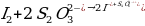
The Purpose of the Experiment
This experiment was to demonstrate the effect of non-reacting ions on the rate of the reaction, this is called primary salt effect. Since the iodine clock reaction was used, the reverse reaction of iodide oxidation was the reduction of iodine to iodide ion with thiosulfate anion preventing the formation of the triiodide ion. The auto-oscillatory nature of this process was determined by iodine oxidation as a rate-determining reaction in which the released free iodine was immediately reduced by thiosulfate, and therefore its activity on starch, the appearance of the blue coloring of the solution, could not be observed if the thiosulfate ion remained in the system. In other words, the blue color appeared as soon as thiosulfate ions were used up. In order to speed up the process, a pseudo-first-order method of reaction was used because the iodide concentration is much higher than the persulfate concentration.
d – where kapp refers to the apparent rate constant of persulfate, ∆x characterizes the concentration of thiosulfate, and ∆t shows the time elapsed from the beginning of the experiment to the appearance of blue staining.
The change in the reaction rate — the decrease in the time for the blue color to appear was due to an increase in the amount of electrolytes with the salt effect. On the contrary, the solubility of some low-soluble salts increases in the presence of other soluble salts that do not have common ions with them. Thus, increasing the concentration of potassium nitrate in the experimental series should have reduced the time of appearance of the blue coloration of the starch-iodine. Thus, the essential purpose of the present experiment was to try to evaluate the applicability of the primary salt effect to the iodine oxidation and reduction system, iodine clock, in the presence of negatively charged nitrate ions.
Pre-Lab Calculation
Due to lack in glassware, the volumes of prepared solutions were adjusted to be suitable with available volumetric flasks.
Sample Calculation
250 mL of 0.1M KI.
Same method was applied to calculate the amount of
1L of 0.001M HCI and 1×10-5 M EDTA
MiVi = MfVf (of HCl, initial Molarity of HCl is 12.1M – available)
Reagent Table
Experimental Molarity of added solutions
Sample calculation
Used same above method to calculate experimental molarity of added solution
Data
Each flask was added 1mL starch to perform as indicator when free iodine molecules float in solution. K2S2O8 was added last, and the timer started counting.
Table: Time of each flask took to change color since the first drop of K2S2O8
Data Analysis and Discussion
- The first three flasks did not have the involvement of the electrolyte KNO3, but they were meant to compare the speed of reaction with differences in the amount of K2S2O8.
- The rest 6 flasks, the amount KNO3 was increasingly added and the amount of K2S2O8 added was kept the same for 6 of them. The purpose of this was to compare the rate of reaction when amounts of reactants the same but different in amount of nonreacting ions.
- Solvent HCl-EDTA was added with different amount to maintain the approximately equal total amount of solution in each flask. (100 mL solution total)
- Discussion using KNO3: Without KNO3 added, the reaction took a long time to have blue color appear, which meant the rate-determing step did not happen quickly to produce iodine molecules. Again, the blue color appeared when all the thiosulfate was used up reacting with iodine. The time between the first three flask different due to the difference of K2S2O8 added. The reaction happened faster when K2S2O8 was added with higher amount because there were higher possibilities of collision between reacting molecules. Addition of KNO3 helped the reactions happen at a higher speed, but also considering the amount of K2S2O8 was added.
- There were some be source of errors which caused the unnecessary fault in this experiment. This could be human error due to pressing the timer as soon as the reactant started to interact with each other, this source could be easily determined as human response time. Another source of error could be the inaccuracy in weighing the reagents could lead to different molarity of each solution.
Conclusion
The experiment Primary Salt Effect in Kinetics of Ionic Reactions was to determine the rate of reaction when adding an ionic reagent (KNO3). This was proved that adding electrolyte affects the ionic strength, if the reacting ions have the same charges, the rate constant increases, if they have different charges, the rate constant will decrease, and if there is any neutral reactant, the rate constant will be independent of ionic strength. In general, this experiment was successful because ten flasks with different ratio of components responding correctly to predicted trend of the rate of reaction.
References
Amdur, I., & Hammes, G. G. (1966). Chemical kinetics. McGraw-Hill Books Co.
Indelli, A., & Prue, J. E. (1959). 23. Kinetic salt effects in the reaction between persulphate and iodide ions. Journal of the Chemical Society (Resumed), 107-111.
Knudsen, O. M., & King, C. V. (1938). The oxidation of iodide ion by persulfate ion. V. The rate at low ionic strength. Journal of the American Chemical Society, 60(3), 687-691.