The chemical element Silver has a chemical symbol Ag and atomic number 47. Its main characteristics are; high thermal conductivity, malleability, reflectivity, high electrical conductivity, soft, white and lustrous metal. It occurs naturally as native silver, as electrum (alloy with gold) and other metals. In addition, Silver may occur in minerals such as chlorargyrite as well as argentite. Approximately, 80% of silver was produced as a by-product of another metal refining such as copper, gold, lead and zinc. Silver is a valuable precious metal used to make an ornament, jewelry, high-value tableware and currency (Butts & Coxe 1967). It has recently been used widely in industries as electrical contacts and conductors and in chemical reactions as a catalyst. Other uses include photographic film, medical, dental amalgam, disinfectants and many more. Commercial significant minerals of silver
The main silver-bearing minerals are acanthite and its higher temperature forms argentite, electrum, tetrahedrite, Pyrargyrite and native silver. Silver combines with many elements, it, therefore, occurs as an essential element in over 100 minerals and it is a minor constituent in many more. Silver bearing-fahlores are silver-bearing ores of the volcanogenic deposit type. They consist of Fe and Ag bearing tetrahedrite that usually contains Zn admixture. There are three varieties of Fe-freibergite, Ag-Fe-tetrahedrite and Ag-tetrahedrite with the predominance of Ag and Fe. Silver bearing tetrahedrite is heterogenous in both deposit types but more heterogenous in sulphostibnite deposit types. The group of high silver Fe-freibergite is pertinent only to sulhostibnite deposit type having two or three known mineral generations and serves as an indicator for high silver ore (Purcell & Peters 1998, pp. 539–546).
Other widespread silver minerals are canfieldite from tin-silver polymetallic deposit type and selenocanfieldite, both hosted by galena ore. Pyrargyrite is known from both deposit types and usually contains an admixture of 0.1 to 0.9% and sometimes Fe, Zn and Se 0.1 to 0.4% weight. It also has some excess silver in 0.01 to 0.31% weights. In addition, silver is commonly formed in a wide variety of hydrothermal deposits and is associated with gold and the base metals such as copper, lead and zinc (Addicks 1940). Most deposits in which silver are the main economic mineral are epithermal quartz veins of five element (Ni-Co-As-Ag-Bi) veins. Epithermal and mesothermal gold deposits also harbor silver as a by-product. Metallurgy of silver The recovery of silver from its ores involves roasting the ore in the furnace. This converts the sulfides to sulfates and the metallic silver is precipitated. A number of metallurgical processes have been used to extract silver from its ore of other metals. During the amalgamation process, liquid mercury is added to the crushed ore and an amalgam of silver-mercury is formed. Mercury is then removed from the amalgam by distillation leaving silver metal. In lixiviation methods, the ore is dissolved in a solution of salt such as sodium cyanide. Silver is precipitated when the solution is brought in contact with metallic aluminum or zinc turnings as illustrated;
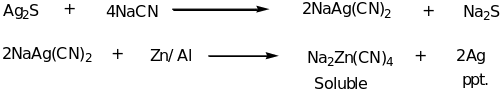
The parkes method is used in separating silver from lead or copper ores. Silver obtained from metallurgical processes is impure. The impure silver is usually associated with unchanged ore, other metal which are produced in similar methods, non-metals such as silicon, carbon as well phosphorous. Other impurities suggested are flux or residual slag. Impure silver is refined by electrolytic methods or by cupellation methods which involves removing impurities by absorption or vaporization. In Cupellation method, the impure silver is heated in oval shaped crucible made of bone ash or cement where a blast of air is passed over the heated molten mass. The impurities are oxidized and removed by the blast air (Butts & Coxe 1967). The pyrometallurgical oxidation process is usually used if the impurities present have a greater affinity for oxygen than the metal itself. Silver may be purified by this method. Common complexes of silver when it is dissolved in seawater The pH of seawater varies over a very narrow range of 8.0 ± 0.7 thus the inorganic composition of seawater shows minimal spatial and temporal variation in chemical constituents. On the contrary, freshwater inflow may cause significant variations of seawater. The most common speciation of silver in sea water involves complexes of chlorine which is relatively high in seawater. Inorganic complexes found in sea water include AgHS0, and chlorine complexes such as AgCI2″, AgCI32′, AgCI due to salinity. Another abundant aqueous species is the silver bisulfide complex AgHS (Purcell & Peters 1998, pp. 539–546). Common complexes of silver when it is dissolved in freshwater Silver is highly toxic in freshwater with a median lethal concentration (LC50) values of about 5 – 60 µg Ag/L.1. It however forms complexes with inorganic cations such as sulfide and thiosulfate. Recent research has indicated the toxicity levels of silver complexes in freshwater. Ag+ forms complexes with soft bases such as sulfur resulting in highly stable organosulfurs complexes. Increase in silver concentration is an indicative application of industrial processes such as mining and the photographic process. The undissociated silver thiosulfate complexes can be converted to chemically inert silver sulfide (Ag2S) which is highly insoluble in water and thereby discharged to environments. Aqueous silver (1) concentration in freshwater is typically very low because the binding power of silver to sulfur is very strong (Purcell & Peters 1998, pp. 539–546).
References
Butts, A. & Coxe, D. 1967, Silver-Economics Metallurgy and Use, Van Nostrand Company, Inc., Princeton, NJ.
Addicks, L., 1940, Silver in Industry, Reinhold Publishing Corporation, New York, NY.
Purcell, T. & Peters, J. 1998, ‘Sources of silver in the environment’, Environmental Toxicology and Chemistry, vol. 17, no. 4, pp. 539–546.