Introduction
According to Kotz and Treichel, the solubility of various substances increases with increases in the temperatures of their surroundings, although this is not always the case. Most often, graphs are used in indicating the relationship between temperature and solubility since it provides a clear view of the trend regarding the two variables. Solubility refers to the quantity of solute necessary to attain equilibrium between the undissolved solute and a saturated solvent at any given temperature. Temperature is a key factor when it comes to solubility of salts substance, and in most cases it is passively correlated with the latter (Kotz & Paul Treichel14).
Being an ionic compound, as Krumhansl indicates, Potassium Nitrate consists of a powerful lattice of ionic bonds (Krumhansl 9). These ionic bonds give the compound a number of physical attributes that certainly affect this study. The compound has both high boiling and melting points because of the powerful linkages, which makes the separation of its molecules extremely energy-intensive. This easily explains the lower rates of solubility of the alkaline earth metals when exposed to lower temperatures. At such temperatures, the cation-anion complex is extremely rigid and the process of separation will require higher activation energy. As the temperature is increased, ionic constituents of the compound collide vigorously because of the high level of energy. As the water bath’s temperature increases, solubility of KNO3 increases since the level of kinetic energy elevated. The process of dissolution is a chemical reaction that involves overpowering of chemical barriers that prevent the attainment of activation energy. The collision between NaCl and KNO3 ions is necessary in order for Potassium Nitrate to dissolve, which ultimately produces energy that allows the occurrence of a chemical reaction.
Purpose
The study was aimed at establishing thermodynamics variables for KNO3 salt in a dissociation reaction involving sodium chloride solution as the solvent. The rate of dissociation of the aforementioned salt varies according to temperature and molar concentration. During the experiment, molar concentrations of Potassium Nitrate were determined, and their values were useful in developing a graphical representation in order to understand the relationship between temperature and solubility. Objectives of the experiment include the following:
- To determine the solubility of KNO3 under a given range of temperatures
- To establish the relationship between temperature and solubility using solubility curve
- To determine how solubility is affected by temperature
Equipments and Material
- Balance
- Clamp
- Crystalline KNO3
- Gas burner
- Graduated cylinder
- NaCl solution
- Rack
- Ring stand
- Spatula
- Test tubes
Procedure
A water bath was prepared in a beaker that was two-third full. The beaker was placed above a heating stand and heated to maintain the water just below its boiling point. There were four test tubes with each containing two-molar solution of Sodium Chloride of volume 9ML, 12Ml, 15ML and 18ML. The stirring process was aimed at increasing the rate at which solvents dissolved, and it was done immediately KNO3 was added into the sodium chloride solution.
19.020 grams of KNO3 salt were weighed and transferred into a test tube containing a two-molar solution of sodium chloride. Sodium, Chloride solution of volume 9 millimeters and the Potassium Nitrate pellets were stirred to mix and heated under a water bath. The heating continued until all the pellets were dissolved, and the volume of the solution in the test tube was measured using a graduated cylinder and later recorded. Afterwards, the test tube was removed from the water bath and allowed to cool. Thereafter, sodium chloride solution of volume 3 millimeters was added into the previous mixture, and a similar process was repeated; the same was done for the third and fourth trials.
Results
Table 1: Laboratory Results.
Discussion
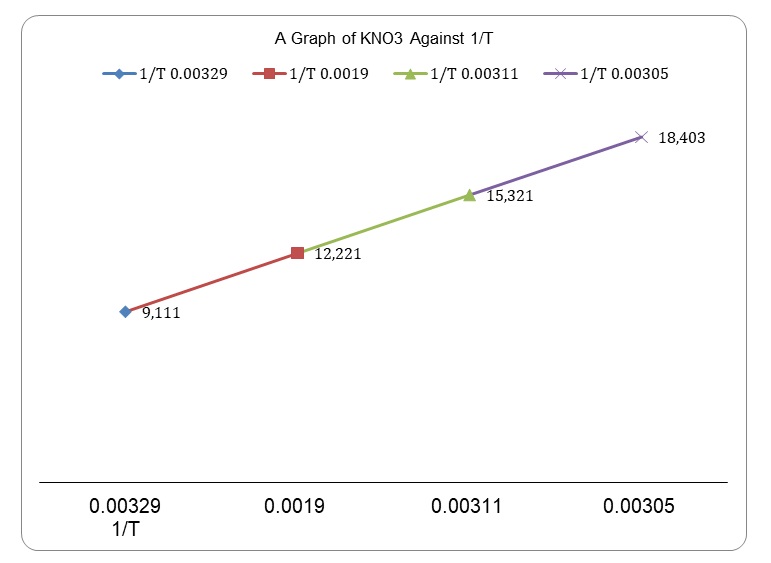
The experiment was aimed at determining the effects of temperature on solubility of salt (Potassium Nitrate) in sodium chloride solution. Its solubility was determined with respect to concentration per volume for a range of temperatures. The objectives were achieved by dissolving a known mass of the solute in sodium chloride under a water bath. Once all the crystals were dissolved, the temperature after the solute was dissolve was recorded since it is considered that this is the point at which a reaction is at point of equilibrium (Krumhansl 19). To have the necessary values, molarities were calculated at each point where all pellets had dissolved. Since the temperature recordings were positive, it was clear that the reaction was exothermic. Having known the solution’s volume and the quantity of KNO3 that was added, it was possible to calculate molarity at every point of equilibrium. [K+][NO3-] was the constant for dissociation, a point where the molarity of potassium nitrate is at equilibrium since the solvent’s stoichiometry is in the ration of 1:1. To determine the relationship that exists between temperature and rate of dissociation, a graph of potassium nitrate against 1/T was plotted, and the two variables demonstrated a linear relationship. An increase in temperature positively correlated with an increase in the quantity of KNO3 that dissolved in the sodium chloride solution, and vice versa. When the quantity of KNO3 that was fully dissociated was 9.111 grams, 1/T was 0.00329. In the second trial, 12.221 grams dissolved at 0.0019, which is a much higher volume than the previous trial. A similar trend is evidenced in the third and fourth test tubes, and correlates to findings from a number of studies (Krumhansl 23).
Table 2: Mass of KNO3 against 1/Temperature.
Conclusion
The study established that the relation between the solubility of KNO3’ and temperature is linear and the variables are directly related. It was also established that, although stirring increases the rate of dissociation, it does not in any way affect the solubility of potassium nitrate. Typically, solutions containing powerful electrolytes, as the case of sodium chloride and potassium nitrate, are fully dissociated (Matson & Orbaek 11). In the experiment, NaCl reacted with KNO3 by exchanging each anion because they have a stoichiometry of ratio 1:1. Having noted that they are among the strongest electrolytes, their constituent ions are fully dissociated at equilibrium. From the empirical study, it will be correct to conclude that temperature is a key factor when it comes to solubility of salts, and the conclusions are supported by Kotz and Treichel works on thermodynamics (Kotz & Treichel 64)
Works Cited:
Kotz, John C., and Paul Treichel. Chemistry & chemical reactivity. 5th ed. South Melbourne, Vic., Australia: Thomson-Brooks/Cole, 2003. Print.
Krumhansl, James L. A Comprehensive Study of the Solubility, Thermochemistry, Ion Exchange, and Precipitation Kinetics of NO3 Cancrinite and NO3 Sodalite.
Washington, D.C.: United States. Dept. of Energy. Office of Science ;, 2003. Print.
Matson, Michael L., and Alvin W. Orbaek. Inorganic chemistry for dummies. New York: Thomson-Brooks/Cole, 2009. Print.