Strontium
Strontium is a vital chemical element found in Group II (II A) of the periodic table.
Fundamentally, together with strontium, the entire members of this group including radium, barium, calcium, magnesium, and beryllium among others have a common atomic structure that makes them members of this group (alkaline earth metals). These members have two electrons on their outer energy level hence the name Group II. Principally, on the periodic table strontium occupies a central position in the group thus it’s either more or less reactive than members of its group. Precisely, strontium is more reactive than all the members above it in the group (e.g. beryllium, magnesium, and calcium), and less reactive than all the members that come below it in the group e.g. barium.
The elemental strontium is chemically denoted as Sr, has an atomic number of 38 and an atomic mass of 87.62 AMU. The name strontium is owed to a Scotland village, Strontia, where the element was discovered. Its existence was unearthed in the year 1790 by one Adair Crawford, an Irish physician. However, it was until two decades later that a pure element of strontium was prepared by one Humphrey Davy, an English chemist.
To this end, using electrolysis of a molten compound (strontium chloride), Davy was able to isolate pure metal of strontium from the compound. However, because of its reactivity in the air, the element rarely exists as a pure element. It has a high affinity for oxygen thus; it exists as a compound of strontium oxide (SEO). Naturally, strontium exists as a mineral compound and the chief sources of commercial interest include strontianite (SrCo3) and celestite (SrSo4).
With regards to commercial production contemporary processes including soda and black ash, methods are known to achieve near-perfect production of strontium compounds from a mineral. To be specific, the duo processes achieve 97 and 98% SrCo3 respectively.
Principally, elemental strontium can exist either as 0 or +2 oxidation states. Under ideal environmental conditions, the later oxidation state is steady enough vital for practical use since its reaction with both water and oxygen is feasible. Strontium boasts a total of 26 isotopes of which 4 happen naturally. The naturally occurring isotopes include “84Sr (0.56%), 86Sr (9.86%), 87Sr (7.0%) and 88Sr (82.58%)” (CHEMICAL, PHYSICAL and RADIOLOGICAL INFORMATION”) are the only stable isotopes.
The rest are artificial isotopes and, are a consequence of radioactivity. Of great importance in radiology are isotopes 89Sr and 90Sr. These emanate from a nuclear fission process of radioactive isotopes (239Pu, 235U or, 238U) after bombardment with a high-energy neutron. This process is depicted by the below nuclear equation:
235U + 1n 89Sr + 90Sr + other fission by-products (CHEMICAL, PHYSICAL and RADIOLOGICAL INFORMATION”)
These two radioactive isotopes are synonymous with the human health fraternity owing to their carcinogenicity. Nevertheless, between the two isotopes, 90Sr is the more potent one thanks to its relatively long half-life (twenty-nine years). 90Sr’s half-life dwarfs 89Sr (51 days) by more than 200 folds. Fundamentally, unlike other radioactive isotopes, strontium decays by emitting a beta together with the creation of a progeny product- virtually a beta emitter. The table below shows a summary of the radiological properties of strontium isotopes.
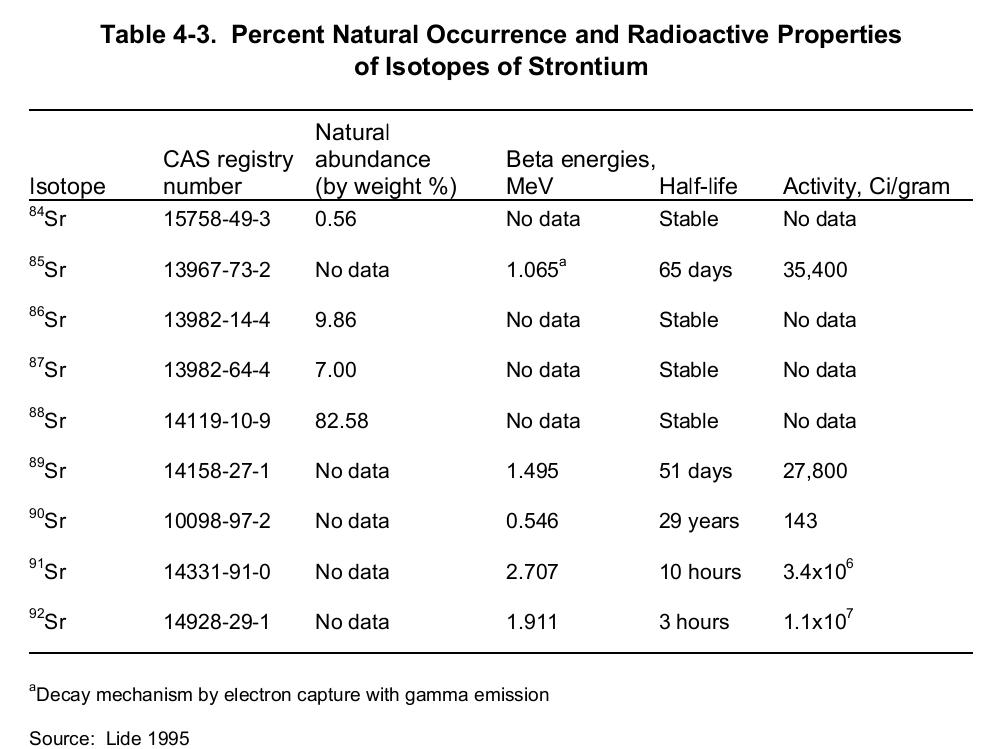
Despite its carcinogenicity, one of the strontium radioactive isotopes is vital in medical studies. Moreover, one major application of strontium is in the production of cathode-ray tubes meant for color television. Also, it is fundamental in “the manufacture of ceramics and specialty glass” (CHEMICAL, PHYSICAL and RADIOLOGICAL INFORMATION”).
Physical Properties
Strontium is a silvery-white, soft element that rapidly reacts in the air (oxygen) to form a thin film of yellow precipitate (SEO). As such, the metal assumes a pale-yellow appearance. Principally, the element assumes a solid-state and has a density of 2.64 g/cm2 at room temperature. However, as it melts the density decreases to 2.375 g/cm3. Moreover, it has a “specific heat capacity of 26.4J/mol/K” (“CHEMICAL, PHYSICAL and RADIOLOGICAL INFORMATION”), and at the melting phase, the heat of fusion is 7.43 kJ per mol.
At the evaporation phase, the heat of vaporization is 136.9 kJ per mol. Importantly, the element has a melting and boiling point of 1050 (7770 C) and 1655 K (13820 C or 25200 F) respectively. Noteworthy, the element is paramagnetic, has an electric resistivity of 132 nΩ.m at 2000 C, and the thermal heat transfers at a rate of 35.4 W/m/K. The table below shows the physical properties of strontium and its compounds.
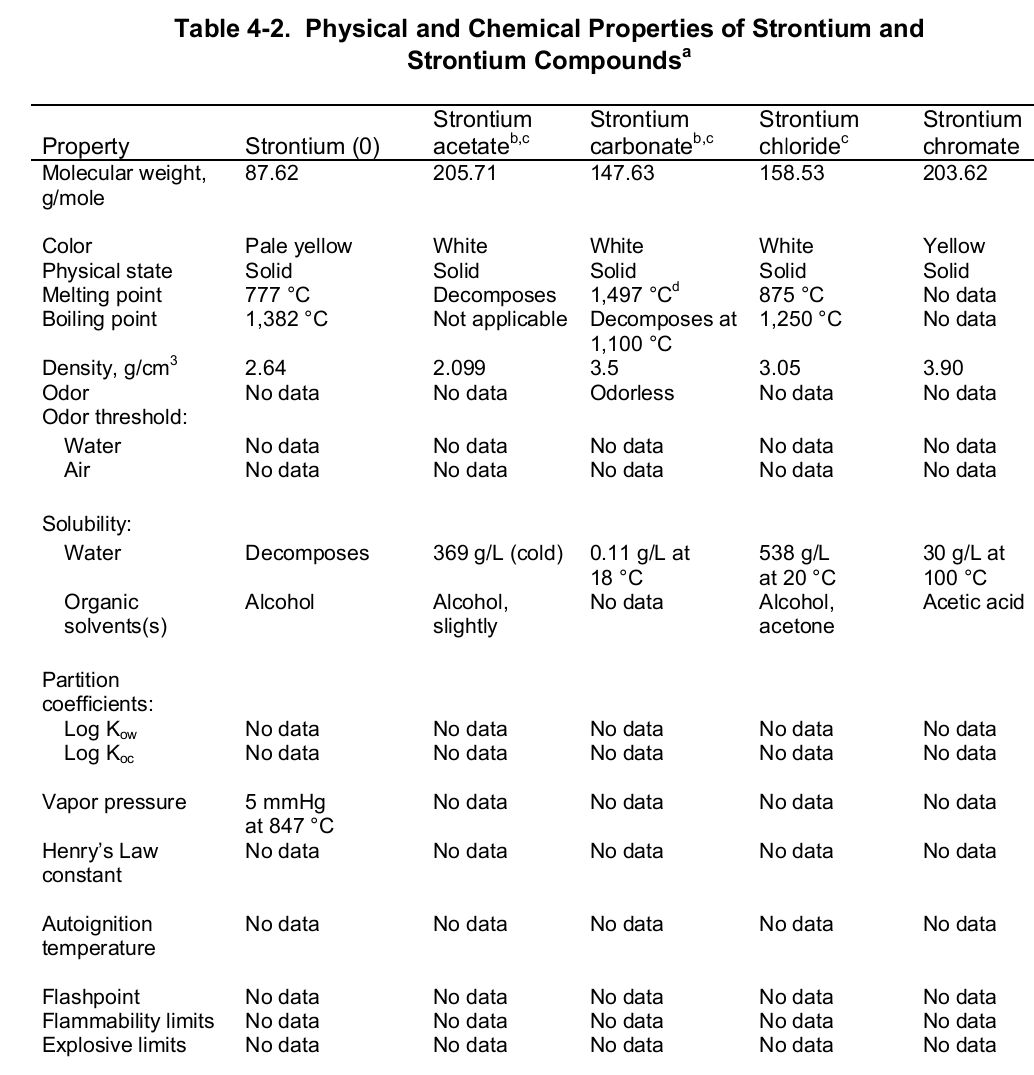
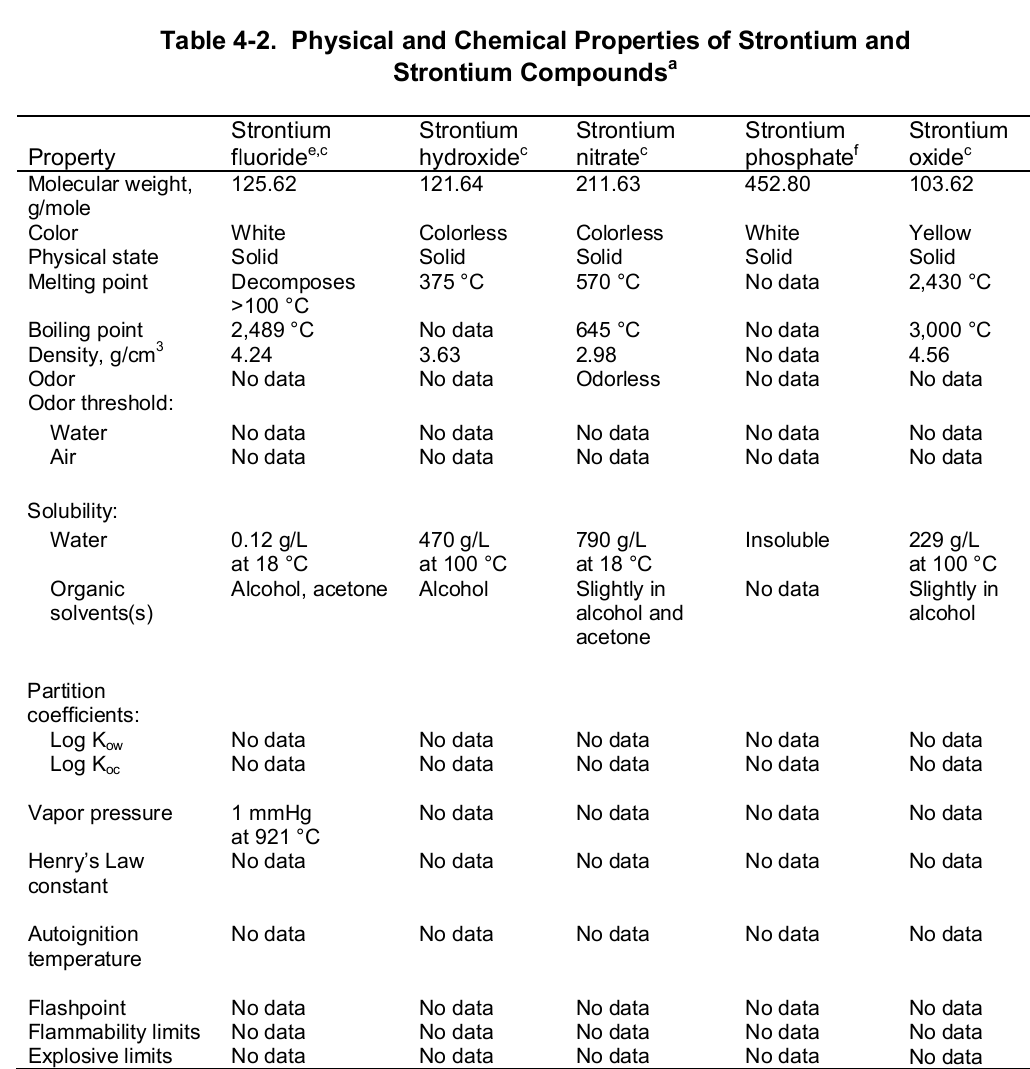
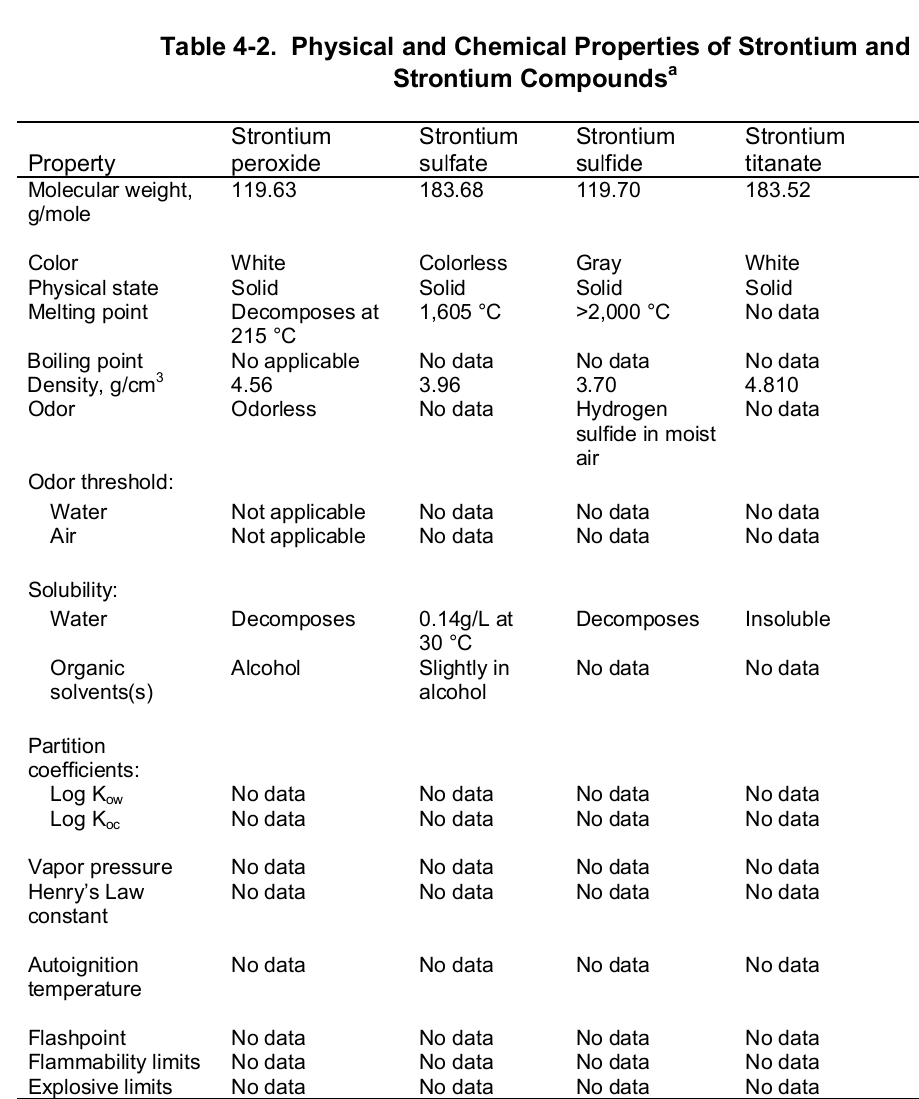
Chemical Properties
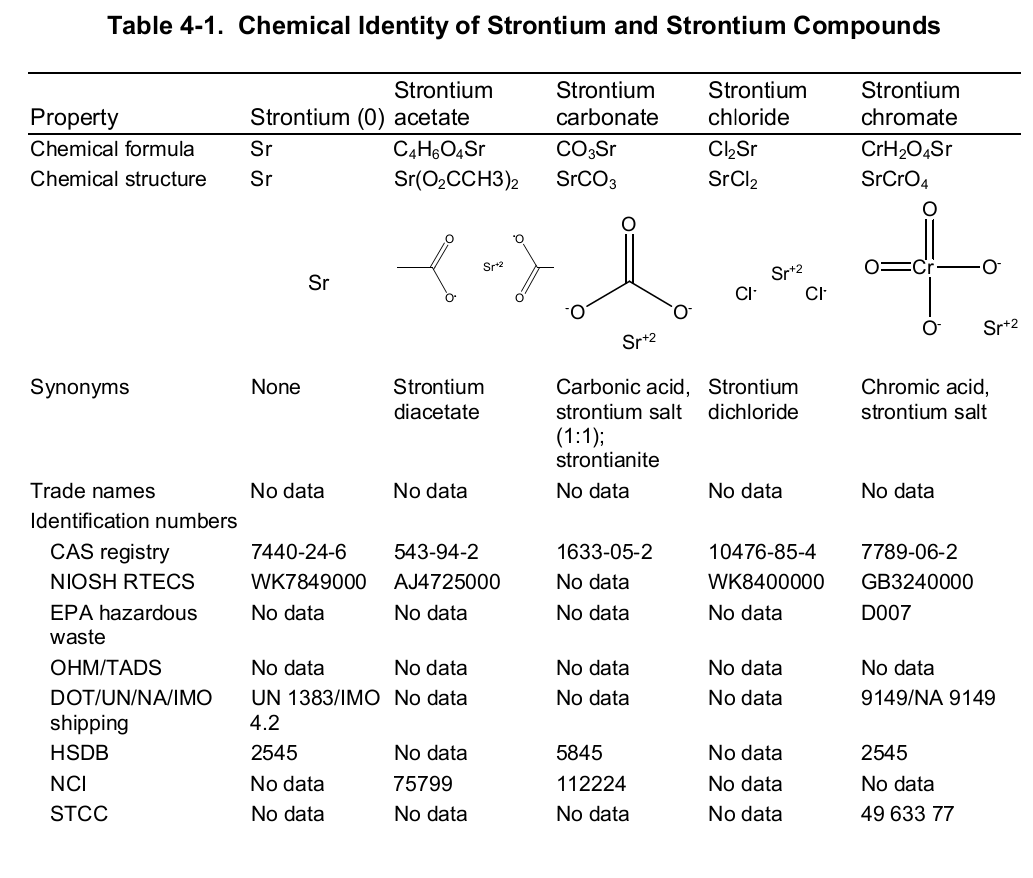
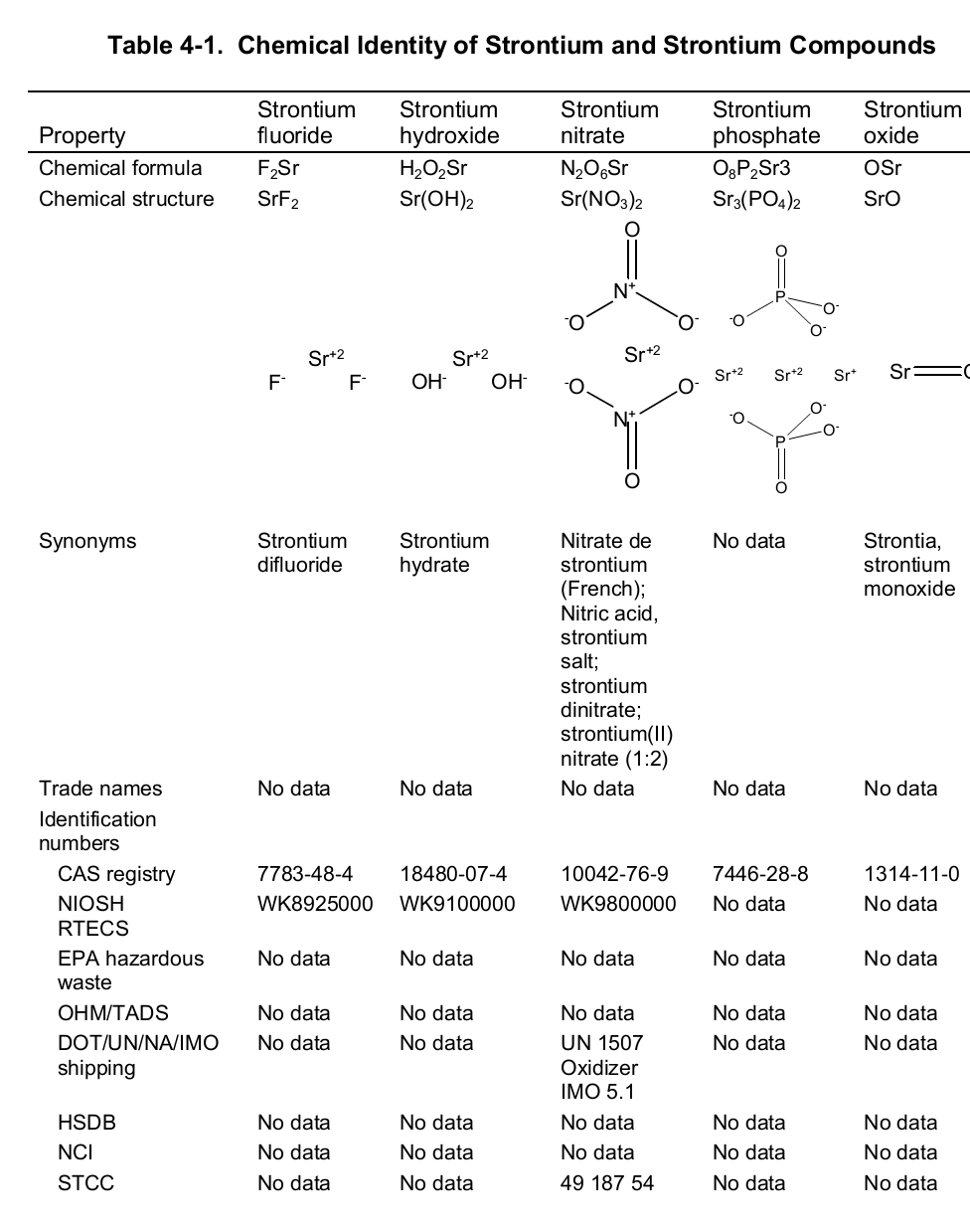
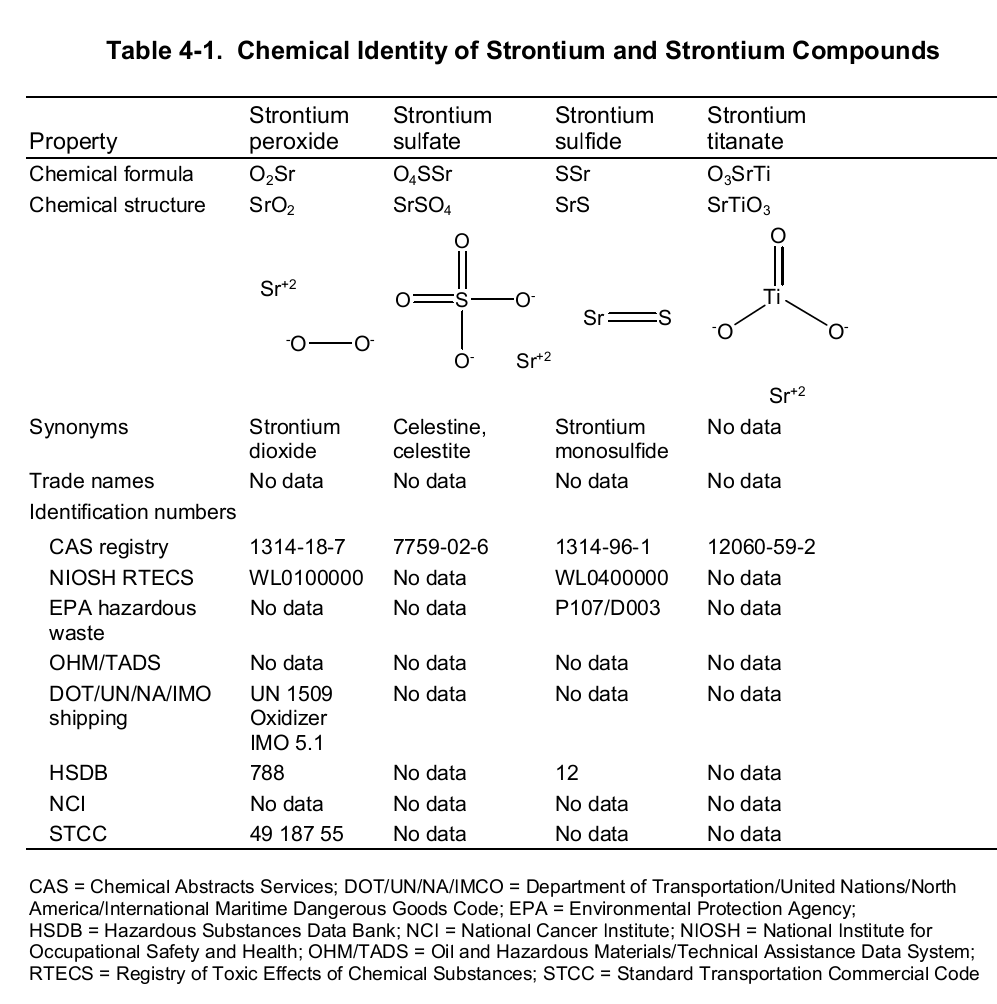
Chemically, strontium is a very reactive element in air thus it reacts spontaneously with oxygen to form strontium oxide which forms a pale-yellow film on the element. As such, pure metals of strontium are always stored in organic solvents to bar this reaction. Also, apart from oxygen the element has a high affinity for both nitrogen and hydrogen consequently forming nitride (Sr 3N 2) and hydride (SrH 2) salts of the same respectively. Moreover, the element reacts with both water and acids liberating hydrogen gas and a salt. It is because of its great affinity for both water and oxygen that the element rarely occurs as a simple compound. To this end, the table below shows the chemical identity of some of its existing compounds.
Works Cited
Chemical, Physical and Radiological Information, Denver: MacMurray, 1999. Print.