Overview
Influenza is a viral infection affecting the upper respiratory tract. It is more common in winter. It results in symptoms such as sore throat, cough, headache, fever, malaise, fatigue, and other non-specific signs. It has long been defined as a significant cause of morbidity and mortality both in adults and recently in children (Izurieta et al. 2000, p.232: Neuzil et al. 2000, p.322). It is estimated that in the United States alone, 20,000 deaths each year are because of influenza epidemics (CDC 1999, p.1). The spread of influenza, an orthomyxovirus, is via aerosols from infected organisms to susceptible hosts. Kilbourne explains that the continuous spread of the virus leads to the production of new viral strains and re-infection, which is the challenge in the development of an effective vaccine (1987, p.334).
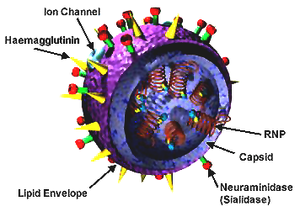
The diagram below shows the configuration of the influenza virus.
Types of Influenza
Scientists classify influenza into three types A, B, and C depending on their nucleoproteins and matrix proteins. However, only type A and B are known to cause diseases in man. Type B has man as the sole reservoir with Type A existing in other hosts known as natural reservoirs like pigs and other mammals (Webster et al. 1993, p.180). The orthomyxovirus has two of the eight segments in its negative single-stranded genome coding for surface glycoproteins (Lamb 1989, p.66: Varghese et al. 1997, p.1108). These glycoproteins are the Hemagglutinin (HA) and Neuraminidase (NA). Hemagglutinin binds to the terminal sialic acid while neuraminidase cleaves the sialic acid. When viewed from outside the virus, the neuraminidases appear like spikes protruding from the viral envelope.
The control of influenza has elicited major developments in drugs and vaccines. Various drugs developed so far have different targets both in the virus and in its replication cycle. One such target is the Neuraminidase, which is an exoglycosidase. It breaks down the hemagglutinin receptor by cleavage of the terminal sialic acid from the adjacent sugar molecule (Gottschalk 1957, p.645: Klenk et al 1955, p.236). This activity of NA is important in the replication cycle of the influenza virus and mainly in the escape of the virus from the envelope. The effect of neuraminidase inhibitors, as will be discussed, is due to their inhibition of the escape of the virus through the envelope and therefore the exit from infected people (Moscona 2005, p.1370)
Drugs that inhibit the activity of NA cause the immobilization of the virus by the upper respiratory tract mucosal secretions (Barnett et al 1997, p.231). Studies have however demonstrated that in vivo replication is not affected by the deficiency of neuraminidase (Liu, & Air 1993, p.784) and the replication occurs at a slower rate (Liu et al 1995, p.243). Examples of Neuraminidase inhibitors include inhaled Zanamivir, inhaled laninamivir, parenteral peramivir, and oral Oseltamivir (Tamiflu) (Sugaya, & Oshasi 2010, p. 132) with other neuraminidase inhibitors being tried for efficacy (Hayden 2009, p.10). This case study looks at Tamiflu (Oseltamivir), its discovery, development, efficacy, safety, and adverse effects in the control of influenza virus infection.
Major and Minor Objectives
The major objective of this case study is to assess the efficacy, safety, adverse effects, and drug resistance of Tamiflu (Oseltamivir) based on the available scientific findings. The specific minor objectives include assessing the efficacy of Tamiflu besides evaluating the documented side effects of Tamiflu, and finally to assess the safety record of Tamiflu. The key issue that this case study will focus on is the development of drug-resistant viral strains. These will be looked at in different sections with appropriate support from existing trials and research conclusions.
Key Issues
Tamiflu (Oseltamivir phosphate), as used in the treatment and prevention of influenza infection, was patented in February 1995 and launched in November 1999. Patented in April 1990 and launched in July 1999 (Kim et al 1997, p.89), its competitor, Zanamivir (Relenza) has found a lot of use in the southern hemisphere. Gilead Science designed Oseltamivir with resemblance to sialic acid bound to NA. Tamiflu is developed from Chinese star anise where shikimic acid is involved in the chemical synthesis of the final drug (Rohloff et al 1998, p.4550: Federspiel et al 1999, p. 270). Its use was marked in the recent H1N1 swine flu infection of 2009. Its development followed other drugs using a sialic acid template (Johnson, & Li 2007, p.108).
Many methods have been developed in the synthesis of Oseltamivir since the development of the prototype (Kipassa et al 2008, p. 815: Fukuta, et al 2006, p.6313.). These have different starting materials, as well as the techniques. The initial drug development utilized shikimic acid as the starting material (Rohloff et al 1998, p.4550) because it closely resembled the targeted site. It is cheap and readily available. Some different starting materials were later tried including the ginkgo leaves. However, the material of choice in the development resulted from the genetic engineering of some bacterial strains of E.coli (U.S. Department of Health and Human Services 2005). This provides the major starting material. To synthesize the final product, Roche, the company of origin of Tamiflu, uses this material.
Two approaches in the synthesis have been defined (Abrecht et al 2004, p.623) with the first making use of Diels Alder. Corey et al successfully used this type of approach in a shorter synthesis process, which yielded approximately 30% over 12 steps (Yeung, Hong & Corey 2006, p.6311). To synthesize oseltamivir, Zutter et al (2008, p.4902) also utilized the second type of approach.
Trost and Zhang later published what was the shortest method in their time of synthesizing oseltamivir (2008, p.3759). This involved the utilization of lactone. There were only eight steps in this method with an overall production yield of 30%. A year later, an eight-step synthesis with a 47% yield was published. This utilized a different starting material (Nie et al 2009, p.3970). Different studies have been conducted looking at the effectiveness of Tamiflu. Some are clinical tries. In one double-blinded, placebo-controlled study, a 74-82% efficacy was observed (Hayden et al 1999, p.1336). This was highly dependent on its use in the subjects under study.
The target of the drug is the Neuraminidase, which it inhibits, resulting in the inability of the virus to exit the host cell thus leading to the subsequent death of the host. In the end, the virus is not able to infect the neighboring cells. Thus, the progression of the infection is controlled. Older antiviral drugs, which inhibit M2, are only active against one of the virus types as compared to neuraminidase inhibitors that are effective on both type A and B. Tamiflu is used in the prevention and treatment of influenza in children older than a year and in adults. The use in children is a major breakthrough in the prevention of influenza since school-going children have been identified as the main source of introduction in homesteads (Longini 1982, p.373). Influenza is also known to exacerbate some chronic conditions such as asthma even mimicking some common illnesses causing delays in treatment and wrong diagnosis (Pattemore 1992, p.332).
Significant issues relating to the use of Tamiflu include the side effects and the proposed development of resistant viral strains. There is a growing concern about the introduction of new antiviral drugs due to the development of drug-resistant viruses (Gubareva et al 2000, p.831). Tamiflu has not been spared. This study focuses on the major issue in light of the importance of the effective control of the influenza virus.
Background Brief
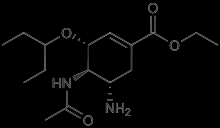
Tamiflu is a neuraminidase inhibitor used in the treatment of influenza. It is indicated that the symptoms have not been present for more than two days. It has also been used in the prophylaxis of children older than a year. The chemical structure is given as.
Mode of Action
The mode of action of Tamiflu involves the inhibition of the neuraminidase on the influenza virus preventing its replication by stopping its exit from infected cells. NA is important in influenza virus replication. Thus, inhibition causes slowing down of the infection (Zambon & Hayden 2001, p.153). Cells neighboring those infected are protected through the action of Tamiflu. Thus, the infection is halted.
Selectivity
Amantadine and Rimantidine, which were the drugs used in influenza treatment, were only active in influenza type A. The development of Tamiflu, which is active on both influenza A and B means it is more appropriate for treatment. The drug also has 100-fold less activity against the sialidase in the human lysosome meaning it is more specific to the virus (Woods et al 1993, p.1477). This also means that the side effect profile is not as significant as with the non-specific drugs (Srange et al 1991, p.704: Monto et al 1995, p.2225).
Indications and Usage
The indications for the use of Tamiflu include treatment of influenza in patients with symptoms for not more than 48 hours. In fact, the diagram below confirms this following the evident 32-hr reduction of the length of time that flu lasts in an adult who has been subjected to Tamiflu medication in relation to placebo.
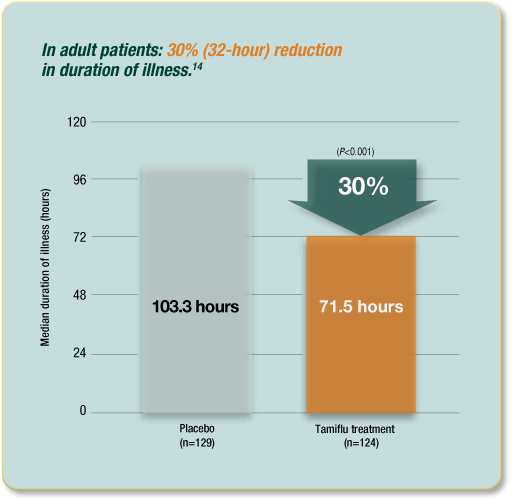
This follows because the efficacy in patients beginning therapy after 48 hours of disease onset has not been established. The drug can also be used in prophylaxis in children who are one year or older. According to the manufacturer, the dosages vary with age, and a dosage of 75mg twice daily for five days is adequate in adults (Roche 2011: Centers for Disease Control and Prevention 1999, p.263). The dosage in children is dependent on their weight.
Side Effects
Limited studies exist, which define the side effects of neuraminidase inhibitors as most of them focus on the reduction of symptoms (Jefferson et al 2009, p.5106: Jefferson et al 2011, Para. 4: Shun-Shin 2009, p.3172). The most frequent side effects are queasiness and barfing. These effects are mild (moderate severity), and maybe reduced if taken with food (Roche 2011: Hayden et al 1999, p.1245). Other serious side effects such as hypersensitivity reactions and neuropsychiatric events have been defined. In clinical randomized controls, bronchitis, insomnia, and vertigo have all been described. The side effects however are dependent on various factors such as the patient’s age, the condition before treatment, and the dosage. Other rare side effects include diarrhea, mild abdominal pain that lasts for some time after drug administration, fatigue, dizziness, headache, and cough. Some studies have demonstrated some of these effects to be as a result of the influenza infection and not necessarily due to the drug administration (Vogel 2002, p.162: Kawai et al 2003, p.427: Mitamura et al 2002, p.950: Machado 2004, p.113: Yamaura 2003, p.890).
Resistance
The development of resistance to Tamiflu has not been significantly demonstrated due to the limited materials necessary in the investigation. In the treatment, the development of resistance has been shown to be less frequent with the use of the drug. More research is necessary to establish the extent of the development of new and resistant influenza virus strains.
Major Issue
The main issue that develops with the use of Tamiflu as indicated above is the development of resistance. Though mild compared to previous antiviral drugs, it continues to pose a challenge in the treatment of influenza virus infection. Observations were made of the development of the strains of influenza viruses that were resistant to Amantadine and Rimantadine with their virulence not decreasing. Resistance is due to the mutation in neuraminidase and haemagglutinin (Zambon 2001, p.150). In a clinical trial of Oseltamivir treatment in children, 18% of them had mutants of neuraminidase isolated that were resistant to Oseltamivir (Kiso et al 2004, p.733). An Oseltamivir-resistant strain of H5N1 was reported in a Vietnamese girl. Nevertheless, she later recovered after the dose was doubled (Mai Le et al 2005, p.1108). In some significant studies, some immune-competent patients were treated with Oseltamivir to determine the resistance rate. Resistance was found to develop in 1-2% of adults (Covington et al 2000, p.326: Gubareva et al 2001, p.527), and about 6% of treated children.
Some findings have shown that resistance is higher in children than in adults. This might be explained by the possibility of children shedding the virus for a longer time compared to adults. It has also been suggested that the reason for this is due to the delay in immune response in children with the initial response being sub-optimal (Moscona 2005, p.1371). In animal studies, the development of resistant viral strains has produced new strains, but these are not as infectious as the wild strains (Mendel, & Sdwell 1998, p.67). They have also been shown to be more pathogenic (Yen et al 2005, p.4068) with no reported transmission of neuraminidase-resistant strains. However, the transmission of viable Oseltamivir-resistant strains was observed in animals (Herlocher et al 2004, p.1628: Yen et al 2005, p.4069). It is therefore important to investigate further its resistance to Tamiflu to assess and prevent further resistance besides developing guidelines associated with its use.
Tamiflu, as shown above by the mode of action, reduces viremia and the duration of illness in patients with influenza. The effect of this is marked with the reduction of symptoms. The interval of disease is shortened by “one to two days” (World Health Organization 2011). It is, therefore, true to conclude that Tamiflu is effective in the treatment of influenza. While looking at the political issues related to the drug, an important consideration is that the drug is recommended for the treatment of patients with serious influenza infections. It has also been given as the drug of choice in severe influenza infections in major health institutions around the world. It is the drug of choice in the widespread epidemics of influenza.
Media’s response to the development, effectiveness, and use of Tamiflu was marked during previous outbreaks of H1N1 where many people were infected with some being treated successfully with the drug. The media described its discovery as a relief and of great importance in taming the epidemic. In the recent epidemics, the New Zealand Times gave clients an article on Tamiflu with definitions and government policies on its usage. The government also provided it over the counter to patients who openly came with the symptoms of influenza.
More research and studies are needed to provide adequate evidence to the use of Tamiflu in influenza treatment. Some of them include the comparison of Zanamivir and Oseltamivir to see their relative side effects and efficacy. More studies are also needed to find out the cost-effectiveness of the drug in clinical practice, and so is further research on the development of resistance in practice. A more focused trial is needed on the efficacy of the drug on resistant strains, especially in children.
Prior to the H1N1 outbreak of 2009, the USA had a large stock of Tamiflu since it was the only oral neuraminidase inhibitor available. Various institutions such as the US Department of Health and Human Service (HHS), The Advisory Committee on Immunization Practice (ACIP), the Australian Therapeutic Goods Administration, and European Medicines Agency (EMA) heralded the development of the drug by saying that it would reduce hospitalizations (HHS 2005, Para. 6: Harper et al 2004, p.56: Roche 2011, Para. 9: EMA 2011, Para. 2). However, the Food Drug Administration (FDA) found that the results of a clinical trial by Kaiser and colleagues did not reduce the complications. They demanded that a statement be made on the drug’s label claiming that this was wrong (Roche 2011, p. 9). Legal claims were made against Roche, the manufacturer of Tamiflu, with the suspicion of making claims contrary to studies. FDA did not approve the use of Tamiflu in the prevention and treatment of influenza based on its findings.
From the above-raised issues, it is clear that, before a product is accepted to be effective, a critical analysis is necessary and this should be carried out by an independent organization with no bias or stake such as a Cochrane review. The use of published reports should be substituted with clinical trials especially where new drugs are involved. The industry has responded with the development of new regulations and a fresh review of the existing studies so that more significance is given to clinical trials. In 2010, Cochrane began a review update using the existing clinical trials instead of the published papers (Harper et al 2004, p.37). This is bound to bring a change in the acceptance of new studies.
Conclusion
In conclusion, the control of influenza viral infection outbreaks is critical in the survival of the human race. Various drugs have been studied with Tamiflu (Oseltamivir) drawing headlines since its discovery and development. The efficacy action, selectivity, and side effects have been looked at with special attention going to the development of resistance to the drug. This has been shown after several reviews to be less significant to the resistance developed by previous antiviral drugs. Therefore, the drug remains effective. In the development and marketing of the drug, legal issues were raised concerning the study methods used to ascertain effectiveness. Despite the drug being recommended by several international bodies, FDA found some inconsistencies in the information given by the manufacturer and studies done. This has led to the review of the methods used to determine drug efficacy.
References
Abrecht, S, Harrington, P, Iding, H, & Karpf, M 2004, ‘Oseltamivir’, Chimia, vol. 58 no. 1, pp. 621-629.
Barnett, J et al. 1997, Susceptibility monitoring of influenza virus clinical isolates to the neuraminidase inhibitor zanamivir (GG167) during phase II clinical efficacy trials [Abstract H-93]. In: Abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy, American Society for Microbiology, Washington, DC.
Centers for Disease Control and Prevention, 1999, Neuraminidase Inhibitors for Treatment of Influenza A and B Infections, MMWR. 48 (No. RR-14): [244-276], Longman Publishers, London.
Covington, E, Mendel, D, Escarpe, P, Tai, C, Soberbarg, K, & Roberts, N 2000, Phenotypic and genotypic assay of influenza virus neuraminidase indicates a low incidence of viral drug resistance during treatment with Oseltamivir. J. Clin. Virol. 18, WVUP, West Virginia.
European Medicines Agency 2011, Summary of product characteristics (Tamiflu 30 mg hard capsule), Web.
Federspiel, M, Fischer, R, Hennig, M, & Mair, H, 1999, Org. Proc. Res. Dev. 3, Routledge, New York.
Fukuta, Y, Mita, T, Fukuda, N, Kanai, M, & Shibasaki, M 2006, Am. Chem. Soc. Routledge, New York.
Gottschalk, A 1957, ‘Neuraminidase: the specific enzyme of influenza virus and vibrio cholera’, Biochem Biophys, Acta, vol. 23 no. 1, pp. 645–646.
Gubareva, V, Matrosovich, N, Brenner, K, Bethell, R, Webster, R 1998, ‘Evidence for zanamivir resistance in an immunocompromised child infected with influenza B virus’, J Infect Dis, vol. 178 no. 1, pp. 1257-62.
Gubareva, V, Kaiser, L, Hayden, F 2000, ‘Influenza virus neuraminidase inhibitors’, Lancet, vol. 355 no. 1, pp. 827–835.
Gubareva, L, Kaiser, L, Matrosovich, N, Soo-Hoo, Y, & Hayden, F 2001, ‘Selection of influenza virus mutants in experimentally infected volunteers treated with oseltamivir’, J. Infect. Dis, vol. 183 no. 1, pp. 523– 531.
Harper, A, Fukuda K, Uyeki, M, Cox, J, & Bridges, B 2004, ‘Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP)’, MMWR Recomm, Rep. 53 RR-6, pp. 1–40.
Hayden, F 2009, ‘Developing new antiviral agents for influenza treatment: what does the future hold?’, Clinical Infectious Diseases, vol. 48 no. 1, pp. 3–13.
Hayden, F, Treanor, J, & Fritz, R 1999, ‘Use of the oral neuraminidase inhibitor oseltamivir in experimental human influenza’, JAMA, vol. 282 no. 1, pp. 1240-6.
Hayden, F, Atmar, R, Schilling, M, & Johnson, C 1999, N. Eng. J. Med 341, Routledge, New York.
Herlocher, L, Truscon, R, Elias, S, Yen, H, Roberts, A, & Ohmit, E 2004, ‘Viruses Resistant to the Antiviral Drug Oseltamivir: Transmission Studies in Ferrets’, The Journal of Infectious Diseases, vol. 190 no. 1, pp. 1627–30.
Izurieta, S, Thompson, W, Kramarz, P, Shay, K, Davis, L, & DeStefano, F 2000, ‘Influenza and the rates of hospitalization for respiratory disease amongst infants and young children’, The New England Journal of Medicine, vol. 342 no. 4, pp. 232–9.
Jefferson, T, Jones, M, Doshi, P, & Del Mar, C 2009, ‘Neuraminidase inhibitors for preventing and treating influenza in healthy adults: systematic review and meta-analysis’, BMJ, vol. 339 no. 3, p.5106.
Jefferson, T, Jones, A, Doshi, P, Del Mar, B, Heneghan, C 2011, Neuraminidase inhibitors for preventing and treating influenza in healthy adults and children – a review of unpublished data.; Cochrane Database Syst Rev 2011: CD008965, Web.
Johnson, D & Li, J 2007, The Art of Drug Synthesis, John Wiley and Sons: Hoboken, New Jersey.
Kawai, N et al. 2003, ‘Effectiveness of oseltamivir on influenza and influencing factors: age of patients, type of influenza virus and timing of initial administration’, Kansenshogaku Zasshi Journal of the Japanese Association for Infectious Diseases, vol. 77 no. 6, pp. 423–9.
Kilbourne, E 1987, Influenza, Plenum Press, New York.
Kim, H et al. 1997, J. Am. Chem. Soc., 119, 681 & J. Med. Chem. 1998, 41, John Wiley and Sons: Hoboken, New Jersey.
Kipassa, N, Okamura, H, Kina, K, Hamada, T, & Iwagawa, T 2008, Org. Lett. 10, John Wiley and Sons: Hoboken, New Jersey.
Kiso, M, et al. 2004, ‘Resistant influenza A viruses in children treated with oseltamivir: descriptive study’, Lancet, vol. 364 no. 9436, pp. 733–4.
Klenk, E, Faillard, H, & Lempfrid, H 1955, ‘Uber die enzymatishe Wirkung von Ifluenza’, Z Physiol Chem, vol. 301 no. 1, pp. 235–246.
Lamb, A. 1989, Genes and proteins of the influenza virus. In: Krug RM, editor. The influenza viruses, Plenum Press, New York.
Liu, C 1993, ‘Selection and characterization of a neuraminidase-minus mutant of influenza virus and its rescue by cloned neuraminidase genes’, Virology, vol. 194 no. 3, pp. 403–407.
Liu, C, Eichelberger, C, & Compans, W 1995, ‘Influenza type A virus neuraminidase does not play a role in viral entry, replication, assembly, or budding’, J Virol, vol. 69 no. 1, pp. 1099–1106.
Longini, M, Koopman, S, Monto, S, & Fox, J 1982, ‘Estimating household and community transmission parameters for influenza’, American Journal of Epidemiology, vol. 115 no. 5, pp. 736–51.
Machado, M et al. 2004, ‘Use of Oseltamivir to control influenza complications after bone marrow transplantation’, Bone Marrow Transplantation, vol. 34 no. 1, pp. 111–4.
Mai Le, Q et al. 2005, ‘Isolation of drug-resistant H5N1 virus’, Nature, vol. 437 no.1, p.1108.
Mendel, D & Sidwell, R 1998, ‘Influenza virus resistance to neuraminidase inhibitors’, Drug Resistance Updates, vol. 1 no. 1, pp. 184–9.
Mitamura, K, Sugaya, N, Nirasawa, M, Shinjoh, M, & Takeuchi, Y 2002, ‘Effectiveness of oseltamivir treatment against influenza type A and type B infection in children’, Kansenshogaku Zasshi – Journal of the Japanese Association for Infectious Diseases, vol. 76 no. 11, pp. 946–52.
Monto, S, Ohmir, S, Hornbuckle, K, & Pearce, C 1995, ‘Safety and efficacy of long term use of rimantadine for prophylaxis of type 1 influenza in nursing homes’, Antimicrob Agents Chemother, vol. 39 no. 3, pp. 2224– 2228.
Moscona, A. 2005, ‘Neuraminidase inhibitors for influenza’, New England Journal of Medicine, vol. 353 no. 13, pp. 1363–73.
Neuzil, M, Mellen, G, Wright, F, Mitchel, F, & Griffin, R 2000, ‘The effect of influenza on hospitalizations, outpatient visits, and courses of antibiotics in children’, The New England Journal of Medicine, vol. 342 no. 4, pp. 225–31.
Nie, D, Shi, X, Ko, K, & Lu, W 2009, J. Org. Chem, Word Press, New York.
Pattemore, P, Johnston, L, & Bardin, P 1992, ‘Viruses as precipitants of asthma symptoms. I Epidemiology’, Clinical and Experimental Allergy, vol. 22 no. 3, pp. 325–36.
Roche 2011, TamifluTM (oseltamivir phosphate) capsules [package insert], Roche Laboratories Inc., Nutley, NJ.
Roche 2011, TAMIFLUH capsules: consumer medicine information, Web.
Rohloff, C et al. 1998, J. Org. Chem, Roche Laboratories Inc., Nutley, NJ.
Shun-Shin, M, Thompson, M, Heneghan, C, Perera, R, Harnden, A, & Mant, D 2009, ‘Neuraminidase inhibitors for treatment and prophylaxis of influenza in children: systematic review and meta-analysis of randomized controlled trials’, BMJ, vol. 339 no. 1, p. 3172
Srange, C, Little, W, & Blarnik, B 1991, ‘Adverse reactions to amantadine prophylaxis of influenza in a retirement home’, J Am Geriatr Soc, vol. 33 no. 2, pp. 700–705.
Trost, B & Zhang, T 2008, Angew. Chem. Int. Ed., 47, Roche Laboratories Inc., Nutley, NJ.
U.S. Department of Health and Human Services 2005, HHS pandemic influenza plan, Web.
Varghese, N et al.1997, ‘Structural evidence for a second sialic acid binding site in avian influenza virus neuraminidase’, Proc Natl Acad Sci USA vol. 94 no. 1, pp. 11808–11812.
Vogel, E 2002, ‘Neuraminidase inhibitors in the management of influenza – experience of an outpatient practice’, Medical Microbiology and Immunology, vol. 191 no. 4, pp. 161–3.
Webster, G, et al. 1993, Evolution and ecology of influenza viruses. In: Hannoun C et al., editors. Options for the control of influenza virus II, Amsterdam: Excerpta Medica.
Woods, M et al 1993, ‘4- Guanidino-2-4-dideoxy-2,3-dehydro-n-acetylneuraminic acid is a highly effective inhibitor both of the Sialidase (Neuraminidase) and growth of a wide range of influenza A and B viruses in vitro’, Antimicrob Agents Chemother, vol. 37 no. 3, pp. 1473–1479.
World Health Organization 2008, UNEDITED REPORT of the 18th Expert Committee on the selection and use of essential medicines, Web.
Yamaura, K & Yoshihara, M 2003, ‘Investigation of the reconsultation rate and pharmacoeconomic evaluation of period of influenza treatment by oseltamivir’, Yakugaku Zasshi – Journal of the Pharmaceutical Society of Japan, vol. 123 no. 10, pp. 887–91.
Yen, L et al. 2005, ‘Neuraminidase inhibitor-resistant influenza viruses may differ substantially in fitness and transmissibility’, Antimicrobial Agents and Chemotherapy, vol. 49 no. 2, pp. 4075–84.
Yeung, Y, Hong, S, & Corey, E 2006, J. Am. Chem. Soc., Roche Laboratories Inc., Nutley, NJ.
Zambon, M, & Hayden, G 2001, ‘(On behalf of the Global Neuraminidase Inhibitor Susceptibility Network), Position statement: global neuraminidase inhibitor susceptibility network’, Antiviral Research, vol. 49 no. 3, pp. 147–56.
Zutter, U, Iding, H, Spurr, P, & Wirz, B 2008. J. Org. Chem, Roche Laboratories Inc., Nutley, NJ.