Introduction
The purpose of this investigation is to verify that the Ideal Gas model accurately predicts gas behavior. The empirical relationships between the pressure (P), volume (V), temperature (T), and amount of a gas (n) are therefore correlated to form the ideal gas law, PV=nRT (Serway & Jewett, 2018). The universal gas constant, R, and its value is 8.314510J/K⋅mol. The equation can be further expressed as ![]()
for a closed system where n and nR are fixed. In this experiment, I will vary the volume of the syringe and observe the change in temperature and pressure due to the volume change.
Method
Equipment
The following is the list of the required equipment for this lab session:
- Computer
- Absolute pressure sensor
- Ideal gas law syringe
- AirLinks (2)
- Analog adapters (2)
- Sensor cables (2)
- Thermistor sensor.
Procedure
- I configured the computer system and the Capstone program.
- The syringe was set at 40 cm3 volume.
- I connected both sensors to analog adapters and each adapter to an AirLink.
- In Capstone, I tapped the Hardware Setup button and chose the components whose ID codes corresponded to those on the AirLinks that were connected to the sensors.
- I connected the audio plug from the syringe to the thermistor temperature Sensor and the hose fitting to the absolute pressure sensor.
- I chose Capstone’s template, which had two graphs where I designated time to the x-axis, one graph pressure on the y-axis, and the other temperature (in K).
- I clicked “record” and then immediately compressed the plunger to bottom out the stop.
- I released the plunger and allowed it to expand by itself. I waited until the temperature and pressure had stabilized before clicking “stop.”
- Eventually, I recorded the syringe’s final volume reading.
Results
The results obtained from the experiment are represented in a data table. Before gas compression, the initial pressure (P1) is recorded from the highlighted region on the pressure graph. The pressure (P2) and volume (V2) are recorded after gas compression.
Before the gas is compressed, the initial pressure (P1), volume (V1), and temperature (T1) are recorded from the highlighted region on the temperature graph. The final temperature (T2) and pressure (P2) recorded are from the peaked highlighted region on the temperature graph. Finally, the volume V0 is determined using the measured values of V1, V2, P1, and P2.
Table 1 – Data for the Experiment
Sample Calculations
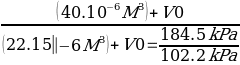
0.0135:0.0135
1:1
Discussion
The experimental values 1 and 2 are similar such that when they are simplified, they give a ratio of 1:1. Similarly, the percentage difference between the two experimental values is 0%. This indicates that the values comply with the acceptable range and the actual value. When the volume of the syringe was instantly lowered in the experiment, the pressure changed by more than a factor of two because of various aspects such as temperature fluctuations and non-ideal gas behavior.
However, as the gas molecules collide with one another and with the syringe walls, they lose energy in the form of heat, and the gas’s temperature begins to reach room temperature. Since the volume of the gas remains smaller than it was initially, the pressure slightly decreases and stabilizes at the new higher value because of the volume decrease (Serway & Jewett, 2018). Finally, when the plunger was released, the gas inside the syringe expanded, which resulted in a decrease in temperature because it started working in the surrounding, causing a lowering of its internal energy.
Conclusion
This investigation confirmed the accuracy of the Ideal Gas model in predicting gas behavior. In the experiment, the gas temperature is the independent variable, the pressure is the dependent one, and the gas volume is constant. Finally, the precise values from the investigation suggest that all pre-lab activities and equipment validation were correctly observed to prevent uncertainties, proven by the ratio and percentage difference values.
Reference
Serway, R. A., & Jewett, J. W. (2018). Physics for scientists and engineers. Cengage learning.