Abstract
In the present work, three types of spectrometric studies (IR, UV, and 1H NMR) were used to qualitatively detect the hexaazatrinaphthylene ligand synthesized according to the scheme of Wu et al. (2019). The results demonstrated consistency with expectations, which means that the resulting substance actually had the same atom groups and bonds as those present in hexaazatrinaphthylene. Based on this, it was concluded that the synthesis was successful.
Introduction
The use of spectrometric analysis is a reliable instrumental method of chemical analytics that allows the qualitative identification of a substance. A comprehensive approach to analysis, in turn, gives increased accuracy to such identification and allows detection of any errors in the synthesis (ATA, 2020). In the present laboratory work, the ligand substance hexaazatrinaphthylene (HAN) was prepared, which is a seven-chain heterocycle with the inclusion of nitrogen atoms, as shown in Figure 1. Three different types of spectrograms were obtained for this substance, including UV, IR, and NMR spectra; remarkably, each showed the expected bands (Wang et al., 2018; García Velázquez et al., 2020). From this, we can conclude that the obtained product was indeed a HAN ligand with a high degree of purity.
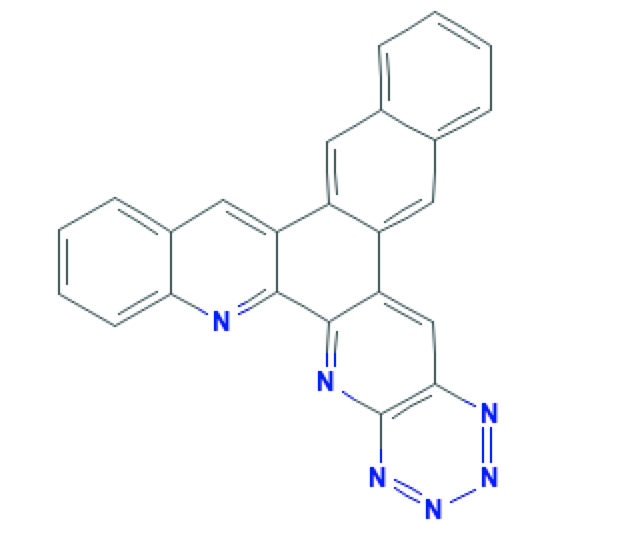
Materials
- 1,2‐phenylenediamine
- a filtration set
- a magnetic stir rotor
- a round bottom flask
- anhydrous methanol
- hexaketocyclohexane octahydrate
Procedure
Hexaketocyclohexane octahydrate (1.25 mmol) was mixed with o-Phenylenediamine (4.12 mmol) in anhydrous methanol (50 mL) using a magnetic stirrer. The solution was subjected to reverse boiling for 240 minutes, after which the suspension was cooled at room temperature. 0.3554 g of the substance, which is about 74% of the yield, was filtered with methanol and dried under vacuum for ten hours. The sample was separated and sent for three different types of spectroscopy.
Results and Discussion
The present work used an experimental scheme to obtain the HAN ligand using the description found in the article. Specifically, the synthesis proposed by Wu et al. (2019) was used to obtain the compound. The purpose of the present study, however, was not only to synthesize the substance but also to qualitatively analyze it using several types of spectroscopy, including UV, IR, and NMR on hydrogen nuclei. For the synthesis, the reaction between 0.4451 g (4.12 mmol) o-Phenylenediamine and 0.3906 (1.25 mmol) hexaketocyclohexane octahydrate in anhydrous methanol was used, as shown in Equation:
The theoretical yield of the reaction, according to Wu et al. (2019) was 94%, and the practical yield of the reaction obtained in our synthesis was 73.9%, according to calculations.
It is noteworthy that the obtained solution of the HAN ligand after crystallization had different shades of yellow, as shown in Fig. 2. There are several possible reasons to explain the variation in color, although it should be primarily emphasized that all of the obtained ligands had a similar color range, indicating the assumed accuracy of the expected compound. The difference in the color of the solid phases indicates, may indicate either the purity of the final product or the density of the intermolecular packing of the compound (Bottari, 2017). We should not exclude the possibility of intercalation of the solvent (anhydrous methanol) into the lattice of the molecule, which resulted in a change in the optical properties of the products.

Conclusion
In the present study, the HAN ligand was synthesized, and then laboratory tested according to the experimental scheme from the article of other authors. UV, IR, and NMR spectrometry performed on hydrogen nuclei demonstrated the presence of the expected characteristic bands, which comprehensively indicates that we obtained the substance we expected. However, an interesting finding in work was the receipt of five different shades of the ligand after crystallization, ranging from creamy white to a saturated red. Several speculations have been made as to the reason for the color change of the ligand in the solid phase.
Experimental Data
The resulting compound was tested using several spectrometry procedures, so the qualitative results of the present experiment were the different types of spectrograms obtained for HAN. Spectroscopy in the UV-visible region demonstrated the presence of a high peak at 300 nm (absorbance 1.672) – this confirms the presence of a bathochromic shift due to the conjugation of benzene rings within the HAN ligand (MSU, 2022). In addition, a characteristic band at the 355 nm position is distinguishable, which may be evidence of an n → π* transition in the -N=N- bonds within the molecule (Cho et al., 2017). Infrared spectroscopy results demonstrate the presence of a band at 3053.8 cm-1, indicating the presence of medium-intensity valence vibrations of multiple bonds in the benzene rings, as well as a 2353 cm-1 band, which is characteristic of diazo compounds, which is the HAN ligand (Merck, 2022). The 1627 cm-1 mark is also prominent, which indicates a C=N- bond in the molecule. Finally, NMR on proton nuclei was used, and several obvious peaks were observed. First, there was a peak at 1.59 ppm that corresponded to covalent bonds between the alkyl carbons of the benzene rings (NMR – Interpretation, 2020). The presence of a peak at 7.28 also reliably indicated the presence of hydrogen bonds to the aromatic ring (Starkey, 2022). A mixture of peaks was also noticeable on the 8.0 ppm and 8.7 ppm bands, which corresponds to hydrogen associated with aromatic rings. In the 13C NMR a peak at 5.38 ppm is also noticeable, which probably indicates the presence of a hydroxyl group in the solvent. The peak at 143.6 ppm may show carbon bonded by a double bond with another carbon and a single bond with nitrogen in the heterocycle. The peak at 132 ppm corresponds to the =C-C= bond within the cycle.
References
ATA. (2020). Spectrometry and spectroscopy: What’s the difference? ATA Scientific. Web.
Bottari, G. (2017, December 02). I prepared a compound that is dark brown in the solid state/solution but forms green crystals once crystallized. Is this color change common? ResearchGate. Web.
Cho, E. N., Zhitomirsky, D., Han, G. G., Liu, Y., & Grossman, J. C. (2017). Molecularly engineered azobenzene derivatives for high energy density solid-state solar thermal fuels. ACS Applied Materials & Interfaces, 9(10), 8679-8687.
García Velázquez, D., Luque, R., & Ravelo, Á. G. (2020). Microwave-assisted synthesis and properties of novel hexaazatrinaphthylene dendritic scaffolds. Molecules, 25(21), 1-33.
Merck. (2022). IR spectrum table & chart. Sigma Aldrich. Web.
MSU. (2022). Visible and ultraviolet spectroscopy. Web.
NCBI. (2022). PubChem compound summary for CID 87114722, hexaazatrinaphthylene. Web.
NMR — Interpretation. (2020). Chem Libretexts. Web.
Starkey, L. S. (2022). 1H NMR chemical shifts [PDF document]. Web.
Wang, J., Chen, C. S., & Zhang, Y. (2018). Hexaazatrinaphthylene-based porous organic polymers as organic cathode materials for lithium-ion batteries. ACS Sustainable Chemistry & Engineering, 6(2), 1772-1779.
Wu, W. H., Huang, M. J., Zeng, Q., Xian, W. R., Liao, W. M., & He, J. (2019). Electrical and magnetic properties of a radical-based Co (II) coordination complex with CH⋯ π and π⋯ π supramolecular interactions. Inorganic Chemistry Communications, 103, 149-153.