Abstract
Auto infectious parasites are known to play an important role in the treatment of autoimmune diseases in human beings. Initially, researchers assumed that these parasites used the same mechanism in the process. However, emerging studies show that auto infectious parasites use different mechanisms in the fight against autoimmunity. This paper seeks to establish the different mechanisms that auto parasites use in the fight against autoimmune diseases in human beings. The paper starts with an introduction identifying the different issues surrounding the topic. This paper focuses on the mechanisms that auto infectious parasites like Strongyloides stercoralis, Hymenolepis nana, Taenia solium, and Enterobius vermicularis use in the fight against autoimmune diseases in humans. The null hypothesis states: Auto infectious parasites such as Strongyloides stercoralis, Hymenolepis nana, Taenia solium, and Enterobius vermicularis do not use different mechanisms to aid in the treatment of autoimmune diseases in humans. On the other side, the alternative hypothesis states: Auto infectious parasites such as Strongyloides stercoralis, Hymenolepis nana, Taenia solium, and Enterobius vermicularis use different mechanisms to aid in the treatment of autoimmune diseases in humans. An overview of these parasites’ pathogenesis in the human body is compared and contrasted. Besides the paper discusses, compares, and contrasts their life cycles to understand the mechanism behind their role in the treatment of autoimmune diseases.
Introduction
Initially, researchers only focused on the negative elements of auto infectious parasites. However, with time, it emerged that these parasites could play a critical role in the treatment of autoimmune diseases in human beings. Nevertheless, even after clearing the initial controversy, researchers could not agree on the mechanisms that these parasites use in the fight against autoimmunity. Some scientists argued that the auto infectious parasites used the same mechanisms in the fight against autoimmunity. The conventional understanding held that these auto infectious parasites activated the production of Th2 cells, which play a critical part in immune response. Most autoimmune reactions are associated with the hyperactive Th1 or Th17 activities. The available literature shows that Th2 is involved in the down-regulation of Th1 and Th17 (Okada, Kuhn, Feillet, & Bach, 2010). This understanding created the notion that all auto infectious parasites used the same mechanism in the fight against autoimmune diseases in human beings. However, with time this understanding was questioned after the emergence of the possibility that different mechanisms could be used in the functioning of these parasites in autoimmunity.
Researchers embarked on different studies to determine the mechanisms that auto infectious parasites use in their role of fighting autoimmunity in human beings. The results of different studies showed that indeed these parasites use disparate mechanisms in countering autoimmune reactions in human bodies. For instance, Strongyloides stercolaris uses a different mechanism to cause immunosuppression and curtail autoimmune reactions. In one case, this parasite suppresses pulmonary allergic reactions using IL-10 to reduce the production of allergen-induced airway eosinophilia and eotaxin. In another case, S. stercolaris activates B-cells, which regulate the activation of Th2 cells that play a critical role in the regulation of Th1 and Th17 (Harris & Cause, 2011). As mentioned earlier, Th1 and Th17 are known to trigger autoimmune reactions in human bodies. Therefore, their downregulation using Th2 plays a critical role in the fight against autoimmunity. Additionally, S. stercolaris is involved in the expression of FOXP3, which plays an important role in the regulation of Th2 to achieve the results discussed earlier in this paper. Therefore, based on this one example, it is clear that auto infectious parasites use different mechanism in the treatment of autoimmune diseases in human beings. Some parasites will trigger a cascade of events to cause immunosuppression while others will counter the reactions that lead to autoimmunity. In other cases, some auto infectious parasites like Enterobius vermicularis are known to trigger autoimmune reactions (Kamradt, Goggel, & Erb, 2005). These varied mechanisms of operation of auto infectious parasites in the fight against autoimmune diseases in human beings form the basis for this paper.
This paper will focus on the similarities and differences between the mechanisms used by auto infectious parasites such as Strongyloides stercoralis, Hymenolepis nana, Taenia solium, and Enterobius vermicularis in the treatment of autoimmune diseases. These parasites all come from different families yet they share the ability to cause autoinfection in their host. Research shows a reverse relationship between autoinfection and autoimmune diseases. An overview of these parasites’ pathogenesis in the human body will be compared and contrasted. Additionally, this paper will discuss, compare, and contrast their life cycles to understand better the mechanism behind their participation in the treatment of autoimmune diseases. The paper will reject the null hypothesis at the end.
Hypothesis
- Ha: Auto infectious parasites such as Strongyloides stercoralis, Hymenolepis nana, Taenia solium, and Enterobius vermicularis use different mechanisms to aid in the treatment of autoimmune diseases in humans.
- Ho: Auto infectious parasites such as Strongyloides stercoralis, Hymenolepis nana, Taenia solium, and Enterobius vermicularis do not use different mechanisms to aid in the treatment of autoimmune diseases in humans.
Background history of auto infectious parasites
Strongyloides stercoralis
The common name for this parasite is threadworm. The sickness caused by this parasite is known as strongyloidiasis. This worm has a heterogonic lifecycle, which means the cycle has both free-living and parasitic phases. The parasite enters the host’s body when an individual walks barefoot on soil or feces containing the infective J3 juveniles. Autoinfection occurs if the juveniles molt twice before exiting the feces. In autoinfection, the first stage larvae, which is know as L1, develops into infective larvae (IL) within the host’s gut. The IL then penetrates the colon wall to re-enter circulation back to the lungs and small intestines to repeat the normal lifecycle.
Hymenolepis nana
This worm is commonly known as the dwarf tapeworm. It is the most common worm, and it enters the host through the ingestion of contaminated foods. The eggs are found in feces of human beings or rodents.
Taenia solium
The common name for this cestode is the pork tapeworm. It is one of the most dangerous cestodes to human beings because it multiplies inside the host.
Enterobious vermicularis
This worm is also known as pinworm. This cestode is well known for its retroinfection capabilities. Infection occurs when the host ingests eggs, which are found in dust particles. Due to the ubiquitous nature of the eggs, this worm is common amongst children.
A discussion on autoimmune diseases
Normally, the white blood cells (WBCs) function by destroying foreign agents in the body. However, at one point the WBCs start attacking healthy and functional body cells due to different reasons. This scenario underscores autoimmune diseases, which may distort the normal cell and organ growth and function. Examples of autoimmune diseases include Lupos, AIDS, multiple sclerosis, diabetes type 2, asthma, and allergies among others.
Autoimmune diseases affect the immune system due to the deregulation of the production of interleukins and especially IL-6. In normal cases, the secretion of IL-6 takes place in the macrophages and T-cells in response to trauma in the body. However, in autoimmune diseases, the dysregulated production of IL-6 receptor affects the activation of T-helper 1 (Th1) and T-helper 17 (Th17), thus affecting the normal functioning of the immune system.
Brief explanation of immune system and immune responses
Macrophages
Macrophages are a form of WBCs, which are involved in phagocytosis. They engulf and digest foreign cells in the body. Therefore, they play an important role in both innate and adaptive immune responses.
B Cells
B-cells are WBCs from the lymphocytes, and they play a central role in humoral immune response. They secrete antibodies when the immune system is activated due to the presence of antigens in the body.
T cells
These cells are a form of lymphocytes, which mature in the thymus. The major differentiating factor of these cells is the presence of T-cell receptors on the surface of any cell where they are present. T-cells occur in different types including helper, cytotoxic, memory, suppressor, and natural killer cells among others. These different types of T-cells function disparately to aid the immune system fight foreign bodies.
Interleukins (IL)
Interleukins are a form of cytokines, which are cellular messenger molecules involved in coordinating communication amongst cells. Normally, interleukins are not stored in the body. Therefore, they are secreted only in the presence of antigens in the body.
Inverse relationship between auto infectious parasites and autoimmune diseases
The conventional understanding held that some microbes were the origin of autoimmune diseases. However, different studies have disqualified this notion by proving the existence of an inverse relationship between auto infectious parasites and immune diseases. For instance, in a research study by Aoyama et al. (2007), it was discovered that the participants with autoimmune liver diseases had lower levels of Strongyloides stercoralis as compared to the control groups, which did not have such parasites. Therefore, it suffices to conclude that auto infectious parasites aid in the management of autoimmune diseases.
Background
Strongyloides stercolaris
Life cycle
The rhabditiform larva of this cestode is passed from the host in feces. This rhabditiform larva can follow the direct development route where it ecdyses two times to become an infective filariform larva. On the other side, the rhabditiform larva can follow the indirect route where it grows into mature males and females before mating to lay eggs, which ultimately hatch into the infective filariform larva. The infective larva is found in the soil and it enters the host via the feet into the blood circulation to the lungs and alveoli. The host coughs and swallows the larva into the gut where it enters the intestinal mucosa of the small intestines. In the duodenum, the parasites molt twice into adult females, where they reproduce via parthenogenesis and the eggs hatch into larvae, which are excreted in the stool.
The mechanism for aiding in the treatment of autoimmune diseases
Strongyloides stercolaris functions by suppressing pulmonary allergic responses. This mechanism occurs via the reduction of allergen-induced airway eosinophilia and eotaxin production using IL-10. After infection, the body is protected from allergen-induced airway hyper-reactivity.
Besides, this cestode functions through adult T-cell leukemia (ATLL), where FOXP3 is expressed in lymphoma/leukemia, which is associated with immunosuppression (Karube et al., 2008). In the expression of FOXP3, Strongyloides stercoralis results in an “intensified helminth infection and greatly increased Foxp3+ Treg numbers” (Taylor, van der Werf, & Maizels, 2012, p. 181). This immune regulation is beneficial as Tregs control TH2-mediated immune pathology.
B-cells regulate the activation of TH2 immune response. B-cells capture antigens using their receptors on the surface of the cell, which stimulates T-cells to produce cytokines. TH2 cytokines regulate the expression of FycR leading to its deficiency, which in turn affects CRP binding. The failure of CRP binding in turn inhibits IgG binding, thus leading to immunosuppression.
HIV-infected patients
Strongyloides stercoralis “hyperinfection has been described in HIV-infected patients as part of the immune reconstitution inflammatory syndrome after starting highly active antiretroviral therapy (HAART)” (Sadlier et al., 2013, p. 2). This aspect shows that this cestode helps in the fight against autoimmune diseases (Lemos, Qu, Laucirica, & Fred, 2003).
Tuberculosis case
S. stercoralis induces a protective immune response against mycobacteria. George et al. (2014) posit, “Co-existent helminth infection is associated with an IL-10 mediated (for filarial infection) profound inhibition of antigen-specific CD4+ T cell responses as well as protective systemic cytokine responses in active pulmonary TB” (p. 1).
Allergies
According to a study by Maggi (2010), most allergic diseases are mainly associated with allergen-specific responses, which are initiated by CD4+ Th2 cells. Besides, another study by Erb (2007) shows, “IL-10 and/or transforming growth factor-b secreted by APCs or regulatory T (Treg) cells in response to a chronic helminth infection, directly interferes with allergic effector mechanisms by inhibiting mast cell degranulation or inhibiting Th2 cell proliferation” (p. 1171).
In a study by Aoyama et al. (2007), it was established that the high levels of S. stercoralis infection led to reduced symptoms of an autoimmune liver disease. Normally, Th1 and Th2 inhibit one another. The infection of S. stercoralis stimulates the production of IL-4, a form of Th2, which inhibits the production of Th1 that is associated with autoimmune reactions in the body.
Hymenolepis nana
Life cycle
Eggs are passed in stool and they enter the host via ingestion from contaminated food. Once in the duodenum, the eggs hatch into oncospheres before penetrating the mucosa. After the penetration, the parasites enter the villi and live in the lymph. The larvae then develop into cysticercoid. After a week, the cysticercoids appear in the lumen of the duodenum and using their well-developed scolex, they attach to the wall for maturity. After maturity, they lay eggs, which are passed in feces.
Asthma case in Ethiopia
Scrivener et al. (2001) carried out a study, which showed that the infection of H. nana prevented “IgE-mediated allergic diseases by blocking effector-cell IgE receptors with parasite-induced specific and polyclonal IgE” (p. 1495). According to the results of the study, the participants infected with the parasite had reduced wheezing as opposed to the control group, which was not infected.
Taenia solium
Life cycle
T. solium has indirect life cycle involving an intermediary host (pigs) and definitive host (humans). Humans ingest the larval stage of the worm via contaminated pork. The cysticercus attaches on the duodenum wall where it matures in around 12 weeks. The adult worm reproduces by self-fertilization to produce zygotes, which cleave into micromeres before becoming a morula. The morula then grows into oncosphere, which lays eggs to be passed in the stool. The intermediary host ingests the eggs, which hatch into oncospheres in the small intestine before attaching on the wall. The oncospheres then penetrate the mucosal membrane of the duodenum to enter circulation where they settle in different areas in the form of cysticerci, which is the larval stage of the worm.
Suppression of macrophage activation
According to Chauhan et al. (2014), T. solium infection inhibits calcium channel activity. Besides, the infection inhibits the production of TLR induced inflammatory cytokine coupled with failed maturation of macrophages. All these activities lead to a cascade of events, which ultimately suppress immune response.
Treg cells
Studies have shown that NOTCH1 is involved in the functioning of Treg where it works together with TGFb to regulate FOXP3. Besides, CD200RIL and STIM2 play critical roles in the development of Treg cells. Treg cells express a molecule, which is encoded by CTLA4 gene and this molecule leads to the overexpression of Th2 effector cells, which inhibit the production of Th1 that is associated with autoimmune reactions (Idris et al., 2012).
Enterobius vermicularis
Life cycle
Eggs are ingested with contaminated food before hatching in the small intestine where the larvae grow into adults after molting twice in the colon. The mature worms mate and the female one releases eggs through the anal opening.
Association with allergies
A study by Boas, Tapia, Rasmussen, and Ronningen (2014) showed no correlation between E. vermicularis infections and food allergens in Norwegian children.
However, another study in Sweden showed that children with food and pollen allergies seemed to have significantly more E. vermicularis infections than the control group without these conditions.
Telesford, Ochoa-Repáraz, and Kasper (2014) showed that the weakening of human MS due to helminthic infection especially E. vermicularis leads to the polarization of Th2, which is responsible for autoimmune suppression by inhibiting Th1. Besides, the infection of these worms enhances FOXP3+ Treg frequency and function. However, this worm triggers autoimmune reactions in pelvic pain caused by intraperitoneal pinworms.
Comparisons
Auto infectious parasites such as S. stercoralis, H. nana, T. solium, and E. vermicularis compare in the mechanism that they use to aid in the treatment of autoimmune diseases in humans. For instance, the parasites show a common trend of suppressing allergic responses and polarization of Th2, which controls the secretion of Th1 that is majorly associated with autoimmune reactions. Additionally, S. stercoralis, T. solium, and E. vermicularis are involved in the expression of FOXP3 and regulation of Th2 cells, which lead to immunosuppression. Similarly, S. stercoralis, T. solium, and E. vermicularis play a critical role in the production and regulation of Treg cells, which are involved in immunosuppression.
Contrasting
S. stercoralis
This worm aids in the treatment of autoimmune diseases through hyperinfection where it helps in reconstituting inflammatory immune responses, especially in HIV-positive individuals. Besides, the worm is involved in multi-functional CD4+ Th1 and Th17 cells, which are critical in allergic reactions. Moreover, S. stercoralis infection leads to the activation of B-cells, which in turn influence the production of Th2 cytokines to regulate FycR leading to its deficiency. Ultimately, this deficiency leads to immunosuppression after affecting IgG binding.
Hymenolepis nana
The available information on the role of H. nana in treating autoimmune diseases is scarce. However, it is known to prevent conditions associated with IgE allergy by blocking IgE receptors and polyclonal IgE.
Taenia solium
This worm suppresses macrophages, inhibits the activity of calcium channels, and plays a critical role in the functioning and encoding of Treg cells, which ultimately lead to the regulation of Th2 (Zeller et al., 2015).
Enterobius vermicularis
Pinworms function the same way as S. stercoralis. However, they are known to cause autoimmune reactions in pelvic pain in intraperitoneal infection.
Discussion
Auto infectious parasites like Strongyloides stercoralis, Hymenolepis nana, Taenia solium, and Enterobius vermicularis use different mechanism in the fight against autoimmune diseases in human beings. For instance, T. solium is involved in the suppression of macrophages by ensuring that they do not mature. On the other side, H. nana is involved in IgE blocking. In this case, IL4 is involved in the production and differentiation of Th2, which affects Th1 levels. Th1 is known to be involved in autoimmune reactions, and thus Th2 counters these reactions. On the other side, Strongyloides stercoralis uses a different mechanism, which involves immunosuppressive B cells. As mentioned earlier, the infection of S. stercoralis activates the production of B-cells. IL-10 and IL-13 are involved in the process where they activate and promote the differentiation of B-cells respectively. Enterobius vermicularis functions in the same way as S. stercoralis. However, the available literature indicates that E. vermicularis is involved in triggering a cascade of systemic autoimmune reactions (Thompson, 2004). Therefore, researchers have split opinion on the role of E. vermicularis in treating autoimmune diseases in humans, given that the parasite causes intraperitoneal pelvic pain. Other parasites use different mechanism in the treatment of autoimmune diseases in human beings. For instance, S. stercoralis is associated with the regulation of Th1 and Th17 cells, which play an important role in allergic reactions.
From the available information as presented in this paper, it suffices to conclude that different auto infectious parasites use disparate mechanisms in the treatment of autoimmune diseases in human beings. However, this subject has elicited different opinions from scientists. Initially, scientists could not agree on whether autoimmune parasites played a significant role in the treatment of autoimmune diseases in human beings. However, the “hygiene hypothesis” cleared the controversies surrounding this issue by noting that indeed auto infectious parasites shaped the immune system in the fight against autoimmune diseases (Zaccone, Fehervari, Philips, Dunne, & Cooke, 2006). Nevertheless, even after the realization that auto infectious parasites shaped the immune system in the fight against autoimmune diseases, scientists could not agree on the mechanisms used in the process. Some argued that these auto infectious parasites use the same mechanism in the fight against autoimmunity in human beings. On the other side, some argued that these parasites use different mechanisms in the process. This controversy formed the basis for this paper as it sought to establish the mechanisms used in by these parasites in autoimmunity. The paper has established that Strongyloides stercoralis, Hymenolepis nana, Taenia solium, and Enterobius vermicularis use different mechanisms in the fight against autoimmune diseases in humans.
Nevertheless, future research needs to focus on finding out the exact functioning of these parasites because a lot is unknown. For instance, while E. vermicularis is known to function by regulating Th1 coupled with playing a critical role in allergic reactions, the parasite is also known to trigger autoimmune reactions leading to pelvic pain. Besides, the functionality of almost all the auto infectious parasites discussed in this paper is not exhausted. For instance, information on the mechanism via which T. solium affects the functionality of CTLA4 gene is scanty (Fumagalli et al., 2010). Additionally, the available information on the functionality of H. nana in countering autoimmunity is scare. Therefore, future research should focus on these issues coupled with involving more autoimmune parasites in their studies. The broader implications for this study would be researchers looking for better ways of how to fight auto infectious parasites in the treatment of autoimmune diseases on human beings. The delicate balance would be how to use these parasites, which are harmful to the health of human beings, in the fight against autoimmune diseases. The way forward would be mimicking the mechanisms, which these auto infectious parasites use and create friendly mechanisms in vivo to elicit the same reactions as the parasites. This way, the mechanism can be used to achieve the same results without using harmful auto infectious parasites.
Therefore, this paper rejects the null hypothesis that auto infectious parasites such as Strongyloides stercoralis, Hymenolepis nana, Taenia solium, and Enterobius vermicularis do not use different mechanisms to aid in the treatment of autoimmune diseases in humans. According to the evidence gathered throughout this paper, it is clear that different mechanisms are used when aiding in the treatment of autoimmune diseases in human beings.
Conclusion/summary
The role of auto infectious parasites like Strongyloides stercoralis, Hymenolepis nana, Taenia solium, and Enterobius vermicularis in the treatment of autoimmune diseases in human beings has been controversial. However, different research studies have been conducted, and scientists now acknowledge that these parasites indeed play a significant role in the fight against autoimmunity in humans. Nevertheless, the mechanisms that these parasites use in the said process have been a controversial topic. The initial consensus held that these parasites used the same mechanism. However, research shows that the mechanisms are different.
This paper has showed that auto infectious parasites like Strongyloides stercoralis, Hymenolepis nana, Taenia solium, and Enterobius vermicularis use different mechanisms in the process of fighting autoimmune diseases in human beings. Some like H. nana use IgE blocking while T. solium suppresses macrophages to achieve the same results. S. stercoralis uses B- cells by activating IL-10 and IL-13. The B- cells are then involved in the production and regulation of Th2, which regulates Th1, thus suppressing autoimmune reactions coupled with causing immunosuppression. E. vermicularis uses the same mechanisms as S. stercoralis. However, E. vermicularis is also known to cause inflammatory autoimmune reactions and thus its usefulness in the treatment of autoimmune diseases is unclear.
Apparently, there is an overarching need for further research on this subject. Future research should focus on determining how these parasites function and come up with mechanisms to replicate the mechanism in vivo. This move will eliminate the need for auto infectious parasites in the human body because they are harmful. The paper has rejected the null hypothesis that auto infectious parasites such as Strongyloides stercoralis, Hymenolepis nana, Taenia solium, and Enterobius vermicularis do not use different mechanisms to aid in the treatment of autoimmune diseases in humans.
Table 1: Summary of the cells involved in the operation of different auto infectious parasites in autoimmunity
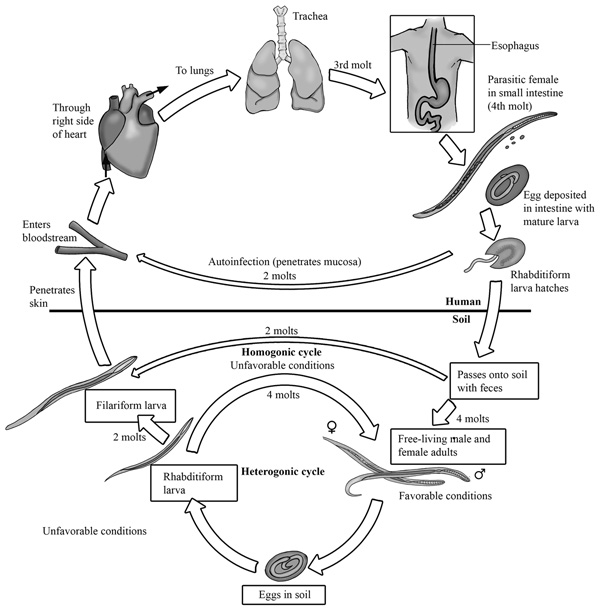
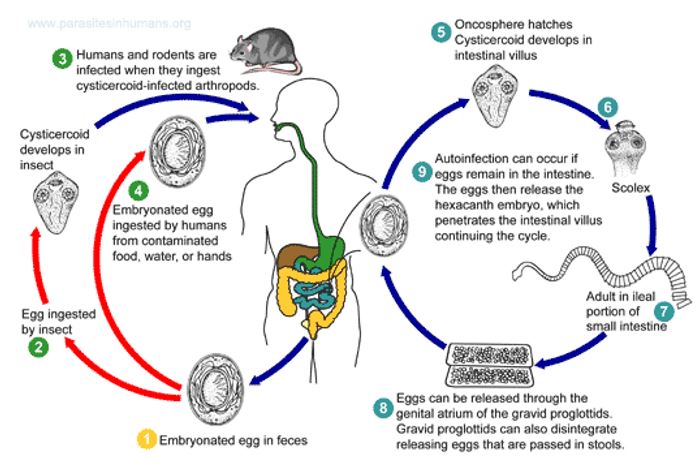
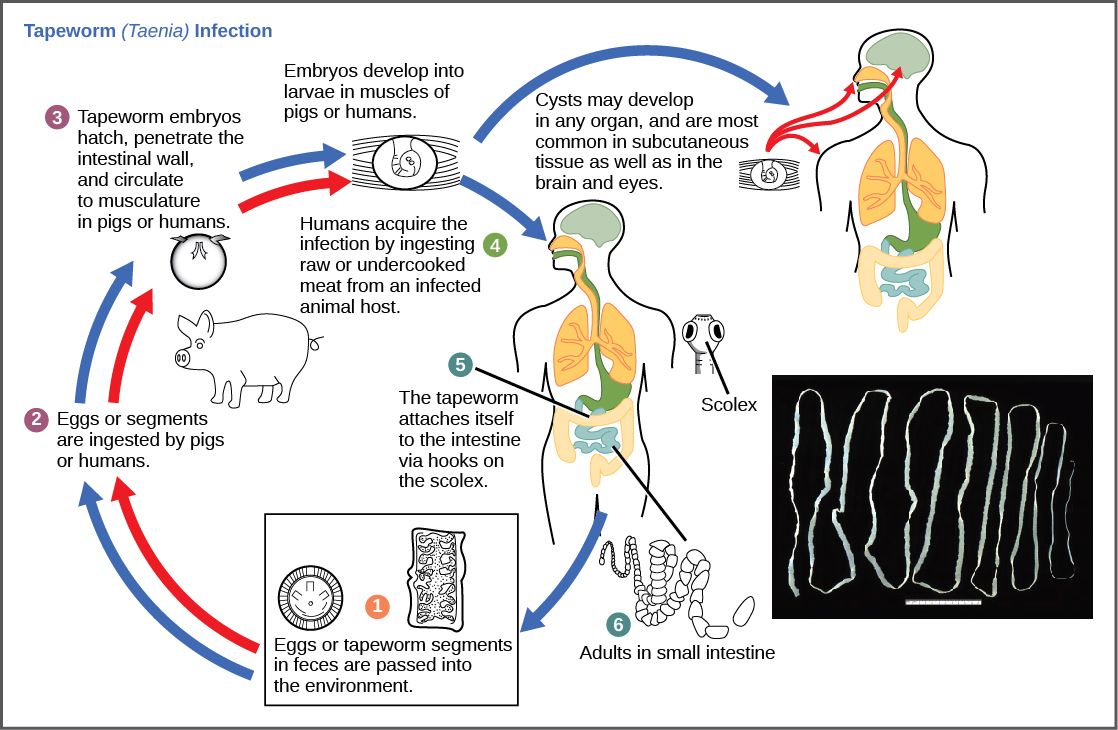
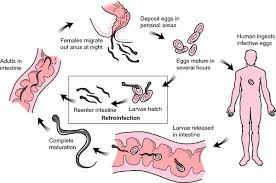
Table 2: Summary of the mechanisms involved in the functioning of different auto infectious parasites in autoimmunity
References
Aoyama, H., Hirata, T., Sakugawa, H., Watanabe, T., Miyagi, S., Maeshiro, T.,…Fujita, J. (2007). An inverse relationship between autoimmune liver diseases and Strongyloides stercoralis infection. American Journal of Tropical Medicine and Hygiene, 76(5), 972-6.
Boas, H., Tapia, G., Rasmussen, T., & Ronningen, K. (2014). Enterobius vermicularis and allergic conditions in Norwegian children. Epidemiology and Infection, 142(10), 2114-20.
Chauhan, A., Sun, Y., Pani, B., Quenumzangbe, F., Sharma, J., Singh, B., & Mishra, B. (2014). Helminth induced suppression of macrophage activation is correlated with inhibition of calcium channel activity. PLoS One, 9(7), 1-11.
Erb, J. (2007). Helminths, allergic disorders, and IgE-mediated immune responses: Where do we stand? European Journal of Immunology, 37, 1170–1173.
Fumagalli, M., Pozzoli, U., Cagliani1, R., Comi, G., Bresolin, N., Cleric, N., & Sironi, M. (2010). The landscape of human genes involved in the immune response to parasitic worms. BMC Evolutionary Biology, 10(264), 1-15.
George, P., Anuradha, R., Kumar, N., Sridhar, R., Banurekha, R., Nutman, T., & Babu, S. (2014). Helminth Infections Coincident with Active Pulmonary Tuberculosis Inhibit Mono- and Multifunctional CD4+andCD8+T Cell Responses in a Process Dependent on IL-10. PLOS Pathogens, 10(9), 1-10.
Harris, N., & Cause, W. (2011). To B or not to B: B cells and theTh2-type immune response to helminths. Trends in Immunology, 32(2), 80-88.
Idris, Z., Yazdanbakhsh, M., Adegnika, A., Lell, B., Issifou, S., & Noordin, R. (2012). A Pilot Study on Cytotoxic T Lymphocyte-4 Gene Polymorphisms in Urinary Schistosomiasis. Genetic Testing and Molecular Biomarkers, 16(6), 488-492.
Kamradt, T., Goggel, R., & Erb, K. (2005). Induction, exacerbation, and inhibition of allergic and autoimmune diseases by infection. Trends in Immunology, 26(5), 260-67.
Karube, K., Aoki, R., Sugita, Y., Yoshida, S., Nomura, Y., Shimizu, Y.,…Ohshima, K. (2008). The relationship of FOXP3 expression and clinicopathological characteristics in adult T-cell leukemia/lymphoma. Modern Pathology, 21, 617-25.
Lemos, L., Qu, Z., Laucirica, R., & Fred, H. (2003). Hyperinfection Syndrome in Strongyloidiasis: report of two cases. Annals of Diagnostic Pathology, 7(2), 87-94.
Maggi, E. (2010). T cell responses induced by allergen-specific immunotherapy. Clinical and Experimental Immunology, 161(1), 10-18.
Okada, H., Kuhn, C., Feillet, H., & Bach, F. (2010). The ‘hygiene hypothesis’ for autoimmune and allergic diseases: an update. Clinical and Experimental Immunology, 160(1), 1–9.
Rajan, T. (2008). Textbook of Medical Parasitology. New Delhi, India: BI Publications Pvt Ltd.
Sadlier, C., Brown, A., Lambert, S., Sheehan, G., & Mallon, P. (2013). Seroprevalence of Schistosomiasis and Strongyloides infection in HIV-infected patients from endemic areas attending a European infectious diseases clinic. AIDS Research and Therapy, 10(23), 1-5.
Scrivener, S., Yemaneberhan, H., Zebenigus, M., Tilahun, D., Girma, S., Ali, S.,… Britton, J. (2001). Independent effects of intestinal parasite infection and domestic allergen exposure on risk of wheeze in Ethiopia: a nested case-control study. The Lancet, 358(9292), 1493-99.
Taylor, M., van der Werf, N., & Maizels, R. (2012). T cells in helminth infection: the regulators and the regulated. Trends in Immunology, 33(4), 181-189.
Telesford, K., Ochoa-Repáraz, J., & Kasper, L. (2014). Gut Commensalism, Cytokines, and Central Nervous System Demyelination. Journal of Interferon and Cytokine Research, 34(8), 605–614.
Thompson, J. (2004). Pelvic pain caused by intraperitoneal Enterobius vermicularis (threadworm) ova with an associated systemic autoimmune reaction. The Journal of Obstetrics and Gynecology Research, 30(2), 90-95.
Zaccone, P., Fehervari, Z., Philips, J., Dunne, D., & Cooke, A. (2006). Parasitic worms and inflammatory diseases. Parasite Immunology, 28(10), 515-523.
Zeller, L., Barski, L., Shleyfer, E., Netz, U., Stavi, V., & Abu-Shakra, M. (2015). Taenia solium in a patient with systemic lupus erythematosus: do parasites protect against autoimmune diseases. The Israel Medical Association Journal, 17(4), 259-60.