Determining the melting point of a compound
Scientists can use the melting point and boiling point to determine a compound’s physical properties. These data can be analyzed and be used to identify a compound or identify its purity. When specifying the composite, a sample to be observed is placed in a melting apparatus. Once its melting point has been established, its data will be matched with other known compounds with the same melting point, hence helping to identify the compound. However, various elements have a common melting point, which necessitates more physical observations such as color to identify the compound correctly. When determining purity, the tester to be observed is placed in melting apparatus, where the accurate reading of the melting point is recorded then compared for the levels of purity.
To determine the melting point, one should have a capillary tube, a small amount of the compound to be tested, a thermometer, and a heating apparatus. After acquiring the apparatus needed for the experiment, a tiny amount should be placed in the capillary tube. The capillary tube will then be attached to the thermometer and be placed in the heating apparatus where both the capillary tube and thermometer can be visible. Then one should proceed into turning on the heat will observing the compound and recording the temperature point where it started melting to the point where it had completely melted. During the experiment, it is advisable to use a dry compound to drop to the bottom of the capillary tube (Qiu, 2018). Also, one should consider low heat when heating the compound so that the melting point doesn’t reach gradually hence missing the correct melting points.
Fatty acids
The biochemistry of lipids revolves around the melting point, chain length of the fatty acid, and saturation level. Complex lipids with unsaturated fatty acids are in the form of liquid (oil) at room temperature. This state is due to bonding, which affects the compound’s structure stability, shape, and charge. The bonding further affects the chemical and physical behavior of the molecule as it affects its interaction. Unsaturated fatty acids tend to create their double bonding at C9 when counting from the COOH end of the bonding structure. The kinks resulting from the double bonding lower the melting points leading to dropped melting points of the unsaturated fatty acids.
Unlike unsaturated fatty acids, saturated fatty acids have single bonding with maximized hydrogens bonded to the carbon. The bonding affects their structure, becoming linear and inflexible hence more tightly stacked. The inflexibility is due to the unavailability of kinks which are caused by double bonding. Molecules that do not have kinks can pack more closely, hence the waxy physical structure of saturated fatty acids in lower room temperatures.
To sum up, saturated fats clog the arteries leading to atherosclerosis. Unsaturated fatty acids can contain one bond hence monounsaturated or many double bonds named polyunsaturated. Fatty acids have 10-24 carbons, with the melting point increasing with carbon numbers. However, the double bonding which creates the difference between saturated and unsaturated fatty acids also contributes to the melting points of the fatty acids. Due to double bonds, saturated fatty acids have higher melting points than unsaturated fatty acids, even if they are made of the same carbon. Good fats should have lower melting points, thus reducing the risk of clog in the arteries. The recommended intake of dairy fats is 3-4 teaspoons of vegetable oil. All fats should contain less than 30% of caloric intake. To reduce dietary fats, one should non-fat or low-fat products, use low-fat cooking methods and choose lean cuts of meat or trim the fat.
Using alkanes and alkenes to determine the fatty acids saturation
Alkanes, alkenes, and fatty acids are made of carbon and hydrogen. There are saturated fatty acids that contain single bonds and unsaturated fatty acids that have double bonding (Sapkota, 2020). Likewise, the alkanes have single bonding replicating the single bonds in saturated fatty acids, and alkenes, which have double bond replicating unsaturated fatty acids, which have double carbon bonding. When bromine is added into alkane solution, it does not react, thus maintaining its color. This is because elements like bromine do not react with saturated hydrocarbon. When the same bromine element is introduced to an alkene that has a double bond, it reacts with the solution adding to the double bond. Applying the information to another hydrocarbon such as fatty acids, one can distinguish between saturated fatty acids from unsaturated fatty acids.
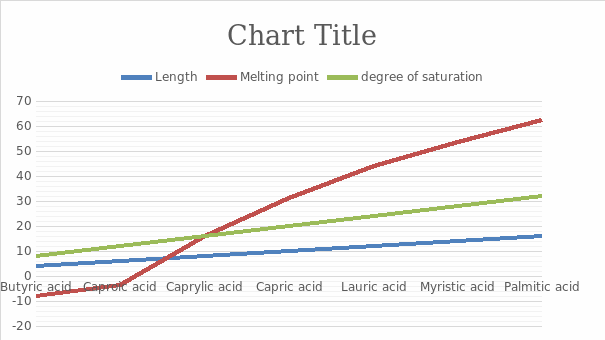
According to the graph, the degree of saturation of the compound increases with the increase of the compound length. This is because the saturation of the compound increases with the rise of hydrogens in the compound. The melting point of the fatty acid increase with the increase of carbons in that compound and saturation. However, the difference between the first elements of the table gives a straight line in the melting point difference while the rest gives a curve. The curve seems predictable hence can be used to determine the melting point of longer compounds.
References
Qiu, F. (2018). Practical Considerations. In Accelerated Predictive Stability, (pp. 75-103). Academic Press.
Sapkota, A. (2020). Saturated vs unsaturated fatty acids- definition, 20 differences, examples. Microbe Notes.