Summary
In the dynamically changing structure and function of the mammalian ovary, growing follicles exhibit a common lineage of development with the OSE layer, and they mutually interact for physical support and exchange of nutrient/hormone/chemical messengers. As mentioned, the stem/progenitor cells of coelomic epithelium derive germ cells differentiating into oocytes and epithelial nests derive the surrounding pre-granulosa cells. In non-mammals oocytes develop in the ovarian cavity layered with surface epithelium. In mammals, OSE assists in the dissolution of the follicle apex and all the underneath connective tissue and helps in releasing the matured ovum.
Further, the mitogens mostly present in follicular fluid influence the OSE proliferation, re-epithelization and tumorigenesis. Given this nature of the intimate relationship between the two integral tissues of the ovary, it was felt necessary to discuss in the following section the key features of mammalian follicle development.
The oocytes and the surrounding cells of follicles constitute the fundamental reproductive unit of the ovary. In young women, the ovarian cortex is largely filled with progressively developing follicles, whereas with aging due to repeated ovulation and atresia most of the follicles are replaced by fibers. Growing follicles migrate deeper in the cortex and subsequently move back to the surface at the time of ovulation. Interestingly, the maximum number of ovarian follicles is found just prior to birth, which gradually declines during childhood and then drops sharply before menopause leaving just ~1000 follicles for the remaining life. Not only does the number declines but the quality of oocytes also deters at this stage, and several structural damages and aneuploidy emerge. According to Carlsson (2008), there are two distinct yet complementary processes that contribute to follicle development:
Oogenesis
The germ cells originating from embryonic ectoderm migrate during early gestation to the genital ridge. During the later gestation period, the primordial oocytes continue to multiply through mitosis, and just prior to birth they enter meiosis, arresting the first meiotic division at the diplotene stage of prophase. Such oocytes are termed primary oocytes. During 20 weeks of human gestation, about 7 million oocytes are produced; of which only 2 million remain at birth and the rest undergo apoptotic destruction.
At puberty, approximately 400,000 primary oocytes still remain, and this is the quota for the reproductive phase of a woman. From this population of oocytes, only 400-500 (1%) follicles mature and ovulate, whereas the remainder becomes atretic. From the onset of puberty to menopause ca. 15-20 primary oocytes are released into the growing pool in each menstrual cycle, completing the meiosis-I and continuing through meiosis-II until metaphase. The second meiotic cycle completes upon fertilization.
Folliculogenesis
This is a sequential process in which the follicles mature from primordial to the pre-ovulatory stage. Two major processes accomplish this development,
- recruitment of the follicle into the growing follicle pool, and
- proliferation and differentiation of granulosa and theca cells.
The first process is regulated by localized paracrine and autocrine factors produced within the ovary itself, whereas the latter is regulated by both localized internal factors as well as the endocrine signals from outside the ovary.
Stages of follicular development
The quiescent primordial follicle (diameter, 30 µm) is constituted by the immature oocyte surrounded by a single layer of squamous (flattened) pre-granulosa cells, and covered by a basement membrane that separates the follicles from the surrounding ovarian cortex. The two events transform primordial follicles to primary follicles,
- granulosa cells start to proliferate and grow into large cuboidal cells, and
- transcription in the oocyte is activated resulting in cell-to-cell paracrine signaling between oocyte and surrounding granulosa.
At this stage, both the cell types start to grow and mutually interact. Primary follicles (diameter ca. 60 µm) are the first level of activation of the recruited primordial follicles. A significant property of granulosa cells is that they secrete mucopolysaccharides around the growing oocytes to form zona pellucida (ZP).
ZP is a thick layer of glycoproteins and proteoglycans between the oocyte and the granulosa layer. The exact source of ZP glycoproteins is not clearly established because both granulosa and primordial oocytes have been shown to produce these proteins. Recent findings by Gook et al. (2007) indicate that three different structural forms of ZP, called ZP1, ZP2 and ZP3, are present in ovaries of most mammalian species.
It was found that in primordial follicles an incomplete ring of ZP comprising mainly ZP1 and ZP3 but not ZP2 is present. Both flattened pre-granulosa cells and immature oocytes are the sources of both proteins. Occasionally, a fraction of the pre-granulosa cells transforms to cuboidal granulosa cells typical of primary follicles. The granulosa cells of such transitory follicles also produce only ZP1 and -3 and continue up to the primary follicles. The oocyte starts to produce small quantities of ZP2 in transitory and primary follicles. Surrounding the basement membrane are the cumulus cells that keeps a gap junction with the oocyte plasma membrane by projecting their microvilli through the ZP.
This allows the bidirectional exchange of nutrients, metabolite precursors, and signal molecules, including the growth factors. As granulosa cells proliferate further and form multiple layers around larger oocytes, the follicles differentiate into secondary or pre-antral follicles (diameter, 100-200 µm). Additional stromal cells from the surrounding region are recruited over the basement membrane to produce two distinct layers, called theca interna and theca externa.
At this stage, the follicles start to obtain their blood supply through microvascular tissue developed between the two theca layers. With the advent of blood supply, circulating FSH facilitates the formation of the multi-lamellate follicle of many layers of actively dividing granulosa cells. The FSH receptors begin to appear on the granulosa cell surface, while the LH receptors are primarily seen in theca cells.
At this stage, all the three ZP proteins are produced by the multilayered granulosa cells through the exterior layer in proximity to the basement membrane exhibits the highest concentrations. A thick band of ZP1 and -3 encompasses the oocytes, while ZP2 appears as a thin layer towards the periphery. The early antral follicle (diameter ~500 µm) contains a fluid-filled sac called the antrum, which contains blood exudates and local secretions including hormones and metabolites. The basement layer separates theca interna and externa from the underneath multilayered granulosa cells. Upon stimulation, with LH the theca cells produce androgen, which is then converted to estrogen within the granulosa cells. A large fluid-filled Graafian or antral (tertiary) follicle represents a mature follicle.
These follicles contain two distinct layers of granulosa cells – the cumulus granulosa cells surrounding the oocyte and mural granulosa cells along the follicle wall. A follicle can attain a size of approx. 20 mm. At this stage, high expression of all the three ZP proteins was noticed in the innermost theca layer in proximity to the basement membrane. As the antral follicle grows the ZP protein concentration starts to deplete from granulosa cells and very little ZP1 is synthesized by the cells of the internal theca. Most of the ZP proteins continue to synthesize in matured oocytes of this follicle. During the estrous cycle of many primordial follicles that start to grow, only one would ovulate in humans and the rest become atretic. Fig. 17 presents an outline of different stages of folliculogenesis.
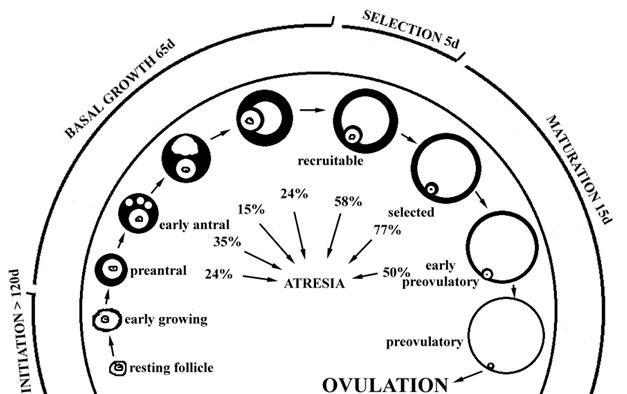
Follicular growth during the menstrual cycle
Analysis of development of large follicles (secondary to pre-antral) in mammalian species shows that their development occurs only at particular reproductive states or during particular times of the reproductive cycle (Fortune, 1994). In humans and other primates, pigs and rats, with menstrual cycle large ovulatory follicle appears only at a particular time rather than randomly during the cycle. A cohort of growing follicles emerges at the early follicular phase and only one follicle continues to grow at the late follicular phase whereas the rest of the cohort regresses. No ovulatory size follicle develops in the luteal phase.
In the other species (cattle, horses, sheep) follicular development involves the development of ovulatory size follicles throughout the cycle, including in the luteal phase. Two to three times during the cycle a “wave” of 3-6 follicles continue to grow until they reach a size close to 5 mm. One of them, slightly larger than the others, continues to grow further while the remainder subordinate follicles regress. Follicular waves also occur during the luteal phase and even during pregnancy and pre-pubertal phase. The emergence of each follicular wave in equine and bovine is associated with a sharp increase in circulating FSH.
Conventionally, the ovarian cycle in women commences with a follicular phase followed by “midpoint” ovulation and then a luteal phase. In between the two consecutive cycles, there exists an inter-ovulatory interval (IOI) which starts with the luteal phase of the preceding cycle followed by the follicular phase of the next cycle. It has been recently demonstrated by Baerwald (2009) that selection of dominant follicle over the subordinate follicles in woman occur not just once during the follicular cycle as perceived earlier, but 1-3 times during IOI which is inconsistent with the observation with bovine and equine.
Distinct major and minor waves of follicular growth were observed in women and mares. Major waves were those in which the dominant follicle was selected for further growth until ovulation, while in minor waves growth and dominance of the follicle were not manifest. In bovine species all waves during IOI were major and only the last one called “ovulatory wave” led to the development of a dominant ovulatory follicle.
The dominant follicles in preceding waves did develop but then these underwent regression, resulting in “anovulatory waves”. In contrast, women and mares exhibited major and/or minor waves during IOI. The final wave of IOI was a major ovulatory wave and the receding waves were either anovulatory major waves or were minor waves. In women too, circulating FSH surge was associated with anovulatory major waves in IOI, but the ovulatory wave was independent of FSH level. Hence, for all practical purposes, the mammalian species exhibit a similar wave pattern of folliculogenesis in the IOI phase.
Local regulatory growth factors/hormones and signaling molecules
Transforming growth factor-β (TGF-β) superfamily in follicle recruitment and development:
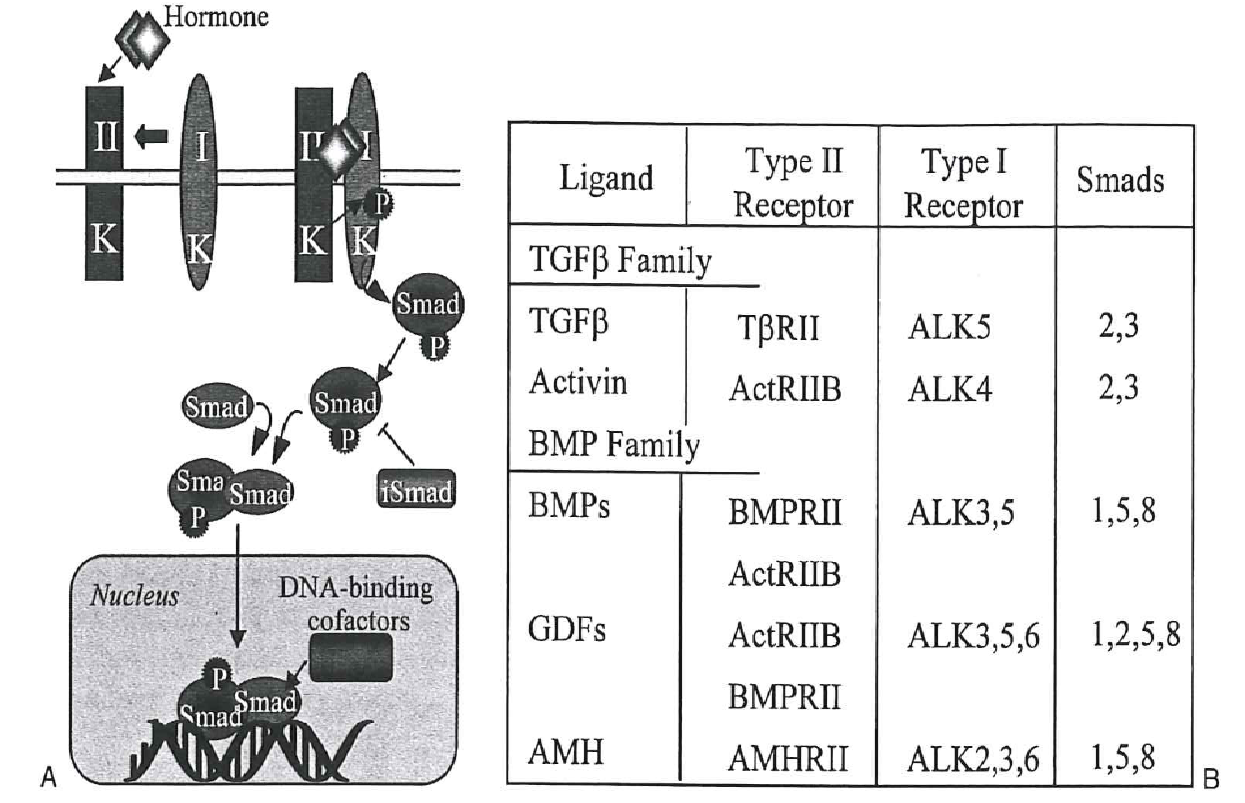
About 40 proteins with similar structures including six cysteine residues belong to this superfamily that influences the growth and development of the follicles (Fig. 18). Broadly, three categories of growth factors viz. TGF-β itself; activins and inhibins; and bone morphogenic proteins (BMP’s) and growth differentiation factors (GDF’s) induce a cascade of signal pathways in growing granulosa through two transmembrane serine/threonine kinase receptors, the activin receptor-like kinase 5 (ALK5; type I) and BMP receptor II (BMPRII; type II). According to Hutt & Albertini (2007), GDF-9 is expressed in the oocytes throughout folliculogenesis.
Deletion of gdf-9 gene in mice resulted in infertility with ovaries in which follicle development was hindered due to lower proliferation of granulosa cells and reduced ability in the assembly of theca cells. Eventually, the oocytes degenerated. GDF-9 signals are mediated through type I and II receptors and SMAD2/3 pathway in granulosa cells and it induces the expression of expansion-related transcripts like pentraxin (Ptx3), hyaluronan synthase (Has2), prostaglandin-endoperoxide synthase (Ptgs2). BMP-15 is a homolog of GDF-9 and is also constantly secreted by oocytes. Mutation in bmp-15 gene arrested follicle development beyond the primary stage and oocytes were lost from granulosa cell clusters.
BMP-15 binds to the type II receptors and functions in conjunction with GDF-9. More recent findings (Li, McKenzie & Matzuk, 2008) supported a hypothesis that these factors contribute to a bidirectional cell-to-cell interaction through gap junctions and paracrine signaling between oocytes and surrounding mural granulosa and cumulus cells in pre-antral follicles. This attribute is advantageous for the oocyte competence and good quality, being regulated by the somatic cells. BMP-15 and BMP-6 protect the cumulus cells from undergoing apoptosis, and thereby oocyte creates a favorable microenvironment of surviving cumulus cells.
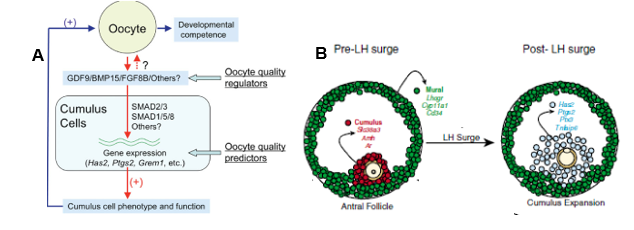
GDF-9/BMP-15 cooperatively regulates cholesterol biosynthesis in cumulus cells. BMP-15 in conjunction with another oocyte-derived growth factor, fibroblast growth factor (FGF8B), regulates cumulus glycolysis. Since mammalian oocytes are deficient in the glycolytic pathway and depend on glycolytic products of adjoining cumulus cells, BMP-15/FGF8B may have significance in oocyte development mediated through the somatic cells. A recent model (Fig. 19 A) depicts how oocyte regulates its own competence by producing growth factors that move into the cumulus cells and then derive functions that are supportive of the oocyte.
During early folliculogenesis, GDF-9/BMP-15 stimulates Kit legend (KL) expression in the oocytes. Together with its tyrosine kinase receptor c-Kit, KL is responsible for the survival, growth and differentiation of primordial germ cells. KL/c-Kit in a paracrine mode down-regulates the expression of GDF-9/BMP-15 and thereby creates a negative feedback loop between granulosa cells and the oocyte. Hutt, McLaughlin & Holland (2006) elucidated the role of KL/c-Kit in folliculogenesis.
Activation of primordial follicle and recruitment is promoted by KL. KL may also be involved in the survival of primordial and pre-antral follicles most likely by evading apoptosis. In the growing follicle, KL also maintains the arrested status of meiosis-I. LH surge decreases the production of KL in cumulus and mural granulosa, a condition that resumes oocyte meiotic division. KL has also been shown to facilitate theca cell recruitment and interaction between theca and granulosa cells in pre-ovulatory follicles, especially the step of estrogen biosynthesis from androgen.
According to Nilsson, Rogers & Skinner (2007), primordial to primary follicle transition is regulated by both inhibitory and stimulatory growth factors. Among the stimulatory factors prominent is FGF2 and KL. One of the inhibitory factors is the Anti-Müllerian hormone (AMH), a member of the TGF-β superfamily which binds to AMH receptor 2 (AMHR2) on the granulosa cell surface and suppresses the proliferation by way of up-regulation of genes under the control of SMAD proteins.
AMH is also reported to partially block the binding of FSH and LH to their receptors and the gonadotropin-induced follicle development is also consequently suppressed. In microarray analysis, AMH was found to negatively interact with different stimulatory growth factors specific to oocyte and granulosa/theca cells, and affect the downstream signaling pathway necessary for follicle transition and development.
Angiogenic factors
The second important component of follicle development is the vasculature for blood supply for oxygen, nutrients, hormones and removal of CO2 arising from respiration. According to Bruno et al. (2009) a wide range of pro-angiogenic and anti-angiogenic factors regulate folliculogenesis and any perturbation leads to atretic degeneration of the follicle at any stage of development. Quiescent primordial to slow-growing preantral follicles do not have a blood supply of their own, but as the antrum develops thecal layer acquires vasculature as two capillary networks each one in theca interna and external.
The most crucial granulosa cell-produced angiogenic factor is the Vascular Endothelial Growth Factor (VEGF), responsible for establishing and increasing the density of the capillary network y assembling the circulating endothelial cells. The assisting pro-angiogenic growth factors that function in conjunction with VEGF are FGF-2 and angiotensin (ANG II). FGF-2 and its receptor, FGFR-2 are respectively produced in inner theca and granulosa cells. These factors enhance the corresponding cells’ proliferative activity and exert an anti-apoptotic response, enabling the production of other angiogenic factors. Besides, FGF-2 is also responsible for primordial follicle activation and recruitment. VEGF also enhances capillary permeability and maintains endothelial cells for micro vascularization. VEGF is produced in oocytes of primordial to primary follicles but in dominant follicles, granulosa and theca cells take over this function.
Another version of VEGF, called endocrine gland-derived VEGF, is produced in granulosa cells of primordial to primary follicles. Angiogenic endothelial cells display positive VEGF receptor expression, and they start to assemble towards the inner theca as initial steps of vascularization. Excess VEGF destabilizes the blood vessels and its level is kept optimal by hCG, LH and FSH. Circulating ANG II regulates oocyte maturation, ovulation and steroidogenesis, and the expression of PDGF, FGF-2 and TGF-β.
Systemic hormones in follicle development
In different mammalian species, the pituitary gonadotropins (FSH and LH) and Insulin-like growth factors (IGF-I and IGF–II) have a considerable impact on the later development of multi-lamellar follicles and on ovulation (Ryan et al. 2008; Campbell, 2009; Madej, Brandt & Einarsson 2009). In fact, the effect of the gonadotropins is seen mostly on the dominant ovulatory follicle when the other sub-ordinate follicles start to regress.
FSH and LH exert their stimulatory effects on proliferation and steroidogenesis by binding to specific G protein-coupled receptors, which in turn causes an increase in cAMP production and activation of the PKA pathway. While the PKA/cAMP transduction pathway is generally considered to be the primary mediator of gonadotropin action, these hormones also activate other signaling pathways that include activation of the Erk and Akt pathways. In addition to gonadotropins other growth factors, mainly IGF’s, stimulate the proliferation of theca and granulosa cells and enhance the ability of gonadotropins to stimulate steroidogenesis in the theca and granulosa cells. In addition IGF’s display a high anti-apoptotic effect primarily on the dominant follicles, while its expression diminishes in the atretic subordinate follicles that are prone to apoptotic degeneration.
These signaling pathways up-regulate the production of progesterone and estradiol in the granulosa cells. In addition, the follicles also express a high level of TGF-β family growth factors like inhibin-A, activin-A and AMH, which interact with gonadotropin-mediated signal transduction. Thus, local growth factors not only modulate the gonadotropin-independent primordial to primary follicle development, but they also regulate LH and FSH action on granulosa and theca cell proliferation at later stages of folliculogenesis. BMP-6 is a factor that augments the gonadotropin action by increasing the FSH induced estradiol and inhibin A production while decreasing the FSH induced progesterone production by granulosa cells.
Another member of these proteins, AMH acts as an inhibitor of the gonadotropin-induced follicle development without affecting steroidogenesis. Further, BMP-15 and GDF-9 exert an inhibitory response on LH-induced thecal steroidogenesis. Increased activity of gonadotropin augmenters (BMP-6) as against decreased activity of gonadotropin attenuators (AMH, BMP-15, GDF-9) determine the success of dominant follicle to proliferation and subordinate follicles to recess.
The rationale of endocrine and developmental factors in ovulation
According to Diaz, Wigglesworth & Eppig (2007), pre-ovulatory antral follicles are comprised of somatic cells that exhibit both endocrine and developmental functions. The cumulus cells in proximity to the oocyte get the developmental signals responsible for the growth and competence of the oocyte via the micro-environment created by adjoining cumulus cells (Fig. 19 B). Prior to LH surge, the oocyte-originated TGF-β signaling pathways regulate the function of cumulus cells and oppose FSH action. Consequently, cumulus specific transcripts, Slc38a3, Amh and Ar, destined for oocyte growth and competence are produced in cumulus cells.
Opposing FSH action enables sustenance of cumulus phenotype in these granulosa cells without being converted to mural granulosa cells. FSH interacts with the mural granulosa beneath the follicle wall and induces transcripts for steroidogenesis (Cyp11a1), ovulation (Lhcgr) and immune function (Cd34). After LH surge, not much change occurs in mural granulose, but the mRNA expression profile of cumulus cells radically changes to cumulus expansion specific transcripts, Has2, Ptgs2, Ptx3 and Tnfaip, which enables freedom of the developed oocytes in the antral fluid for release upon follicle rupture.
References
Baerwald, A.R.(2009). Human antral folliculogenesis: what we have learned from the bovine and equine models. Animal Reproduction, 6(1), 20-29.
Bruno, J.B., Matos, M.H.T., Chaves, R.N., Celestino, J.J.H., Saraiva, M.V.A., Lima- Verde, I.B., Araújo, V.R. & Figueiredo, J.R.(2009). Angiogenic factors and ovarian follicle development. Animal Reproduction, 6(2), 371-379.
Campbell, B.K. (2009). The endocrine and local control of ovarian follicle development in the ewe. Animal Reproduction, 6(1), 159-171.
Carlsson, I.B. (2008). Regulation of Human Ovarian Folliculogenesis In Vitro. Karolinska Instituet, Reproprint AB, Stockholm.
Diaz, F.J., Wigglesworth, K. & Eppig, J.L. (2007). Oocytes determine cumulus cell lineage in mouse ovarian follicles. Journal of Cell Science, 120, 1330-1340.
Fortune, J.E. (1994). Ovarian Follicular Growth and Development in Mammals. Biology of Reproduction, 50, 225-232.
Gook, D.A., Edgar, D.H., Borg, J. & Martic, M.(2007). Detection of zona pellucida proteins during human folliculogenesis. Human Reproduction pp.1–9. Web.
Hutt, K.J. & Albertini, D.F.(2007). An oocentric view of folliculogenesis and embryogenesis. Reproductive BioMedicine, 14(6), 758-764.
Hutt, K.J., McLaughlin, E.A. & Holland, M.K. (2006). Kit ligand and c-Kit have diverse roles during mammalian oogenesis and folliculogenesis. Molecular Human Reproduction, 12(2), 61–69.
Li, Q., McKenzie, L.J. & Matzuk, M.M. (2008). Revisiting oocyte–somatic cell interactions: in search of novel intrafollicular predictors and regulators of oocyte Developmental competence. Molecular Human Reproduction, 14(12), 673–678.
Madej, A., Brandt, Y. & Einarsson, S. (2009). Endocrine dynamics associated with follicle development in pigs: a review. Animal Reproduction, 6(1), 135-143.
Nilsson, E., Rogers, N. & Skinner, M.K. (2007). Actions of anti-Müllerian hormone on the ovarian transcriptome to inhibit primordial to primary follicle transition. Reproduction, 134, 209–221.
Ryan, K.E., Glister, C., Lonergan, P., Martin, F., Knight, P.G. & Evans, A.C.O. (2008). Functional significance of the signal transduction pathways Akt and Erk in ovarian follicles: in vitro and in vivo studies in cattle and sheep. Journal of Ovarian Research, 1:2. Web.