Abstract
Water is a polar solvent hence it is the most commonly used fluid. Apart from the polarity of a solvent in relation to the substance being dissolved, temperature also plays an important role in the dissolution of substances. This experiment expected to establish the relationship between temperature and the solubility of KNO3. The solubility of KNO3 in water and in other salt solutions was determined at different temperatures. It was realized that the solubility of KNO3 increased with a raise in temperature and that the chemical composition of different solvents affected the solubility of KNO3.
Water is the most suitable solvent for dissolving ionic compounds due to its polarity. The polar nature of water allows negative ions from ionic compounds to be attracted to the partially positive hydrogen ion. Ions with positive charges, conversely, are pulled towards oxygen ions, which possess negative charges. Strong forces of attraction hold the oppositely charged ions within molecules. Therefore, energy is required to overcome these forces of attraction. This energy is known as the enthalpy of solution and is denoted by -∆H°soln. Factors such as temperature and the nature of the solvent also affect the amount of energy required to dissolve a compound.
This experiment was aimed at finding the solubility of KNO3 in water as well as in solutions of other salts. It intended to find out the temperature dependence of the solubility of KNO3 in water. The experiment also sought to approximate the heat of solution for potassium nitrate. The final objective was to estimate the molal solubility of potassium nitrate in relation to temperature in solutions containing any of the two ions that made up KNO3.
Procedure
Solubility of KNO3 in Water
About 20g of water was weighed as accurately as possible into a test-tube whose length was eight inches. 9g of potassium nitrate was added to the water, and the test tube was shaken until all the salt was submerged in the water. A coated wire stirrer was then placed in the tube after which a thermometer was inserted in such manner that the bulb of the thermometer did not get in contact with the sides or the bottom of the test tube. It was ensured that the wire stirrer could move upwards and downwards (in the test tube) without upsetting the thermometer. The solution was stirred and heated lightly. The heating stopped when all the crystals liquefied. The flame was removed, after which a beaker containing cold water was placed under the test tube.
The beaker was raised so that the test tube was partially immersed in the water. Thereafter, the solution was stirred vigorously until it became cloudy. The cloudiness was an indication that crystals were starting to develop. The precise temperature at which crystals began to form was noted and recorded as the solubility temperature. The more accurate estimation of the same temperature was determined by reheating the tube and stirring the contents vigorously until the temperature was between 2 and 3 °C higher than the previously recorded temperature. The test tube was removed from the heat, after which the salt was stirred vigorously without cooling in a water bath. The temperature at which the first cloudiness appeared was noted and recorded as the true solubility temperature.
The above procedures were repeated until consistent temperatures were obtained. The purpose of repetition was to check the accuracy of the results. 3g of KNO3 was added to the previously used mixture to make a concentration of 12g. The solubility of the new concentration was determined as previously described. The procedure was repeated to obtain solubility temperatures for concentrations of 15g and 18g. The obtained values were recorded in a table alongside corresponding molal solubility in terms of moles of KNO3 per kilogram of solvent mass (Grossie and Underwood 259).
Treatment of Data
Assuming that the KNO3 in aqueous solution was completely dissociated, then
Ksp= [K+]•[NO3–] = S2 (8) where ‘s’ was the molal solubility. The application of thermodynamic equations to ionic solutions gave the equation
Ln Ksp= -∆Hosoln/R• (1/T)+ C (9) where Ln Ksp was the solubility product constant, -∆H°soln denoted the standard heat of solution for the solute, T was the absolute temperature, R was the gas constant and C was a constant of integration (Grossie and Underwood 259). The molal solubility was determined and tabulated for each of the four temperatures. The values of the parameters in equation 9 were also calculated and tabulated. Thereafter, a plot of Ln Ksp versus 1/T was made. The plot was used to determine the value of ∆H°soln.
A second plot of ∆G°soln (J/Mol) against T (K) was made. The plot was used to obtain ∆H°soln from the slope. The experimental values were then compared to the literature values.
Solubility in Solutions with a Common Ion
It was anticipated that the solubility of KNO3 would reduce in solutions that contained ions that were present in both salts because of the common ion effect. The solubility of potassium nitrate in salts that contained common ions was established using 1 or 2 molal solutions of KCl, NaNO3 and NaCl. The procedure that was used was the same as that in section A. The solubility temperatures of KNO3 in different salts were tabulated. The KNO3 solubility data were plotted alongside the additional solubility data on the same chart.
Results
Table 1: The solubility of KNO3 in water.
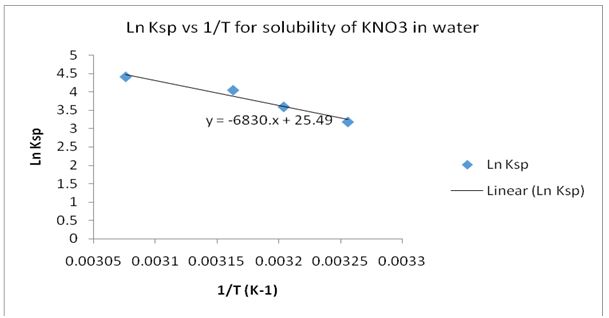
∆H°soln=-R*Slope =-8.3143*-6830 =56,786.669 J/mol =56.787 KJ/mol
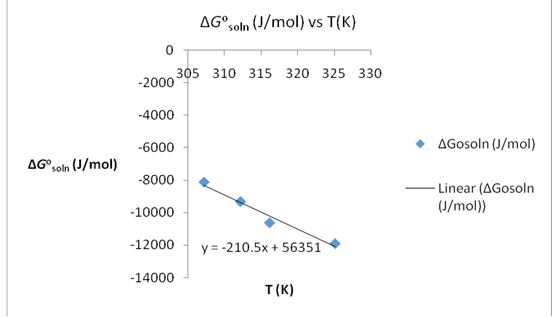
-∆S°soln= Slope
-∆S°soln= 210.5
∆S°soln= 210.5 J/Kmol
Table 2: The solubility of KNO3 in 1M NaCl.
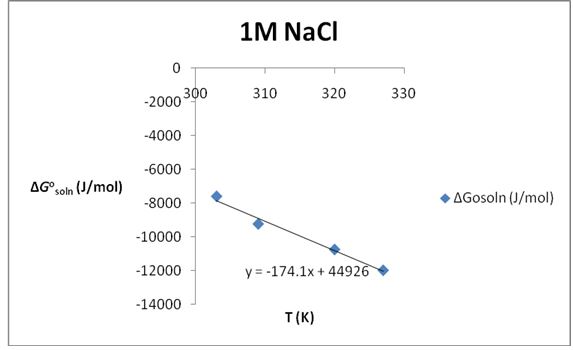
∆S°soln= 174.1 J/K*mol; ∆H°soln=44.926 KJ/mol
Table 3: The solubility of KNO3 in 2M NaCl.
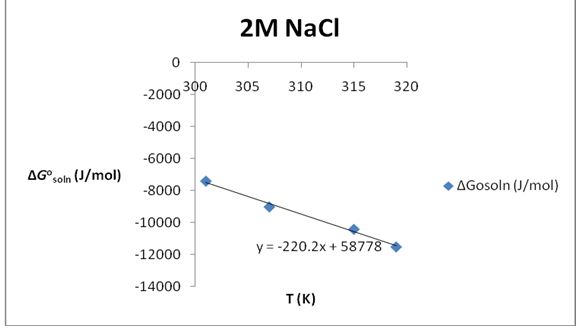
∆S°soln= 220.2 J/K*mol; ∆H°soln=58.778 KJ/mol
Table 4: The solubility of KNO3 in 1M NaNO3.
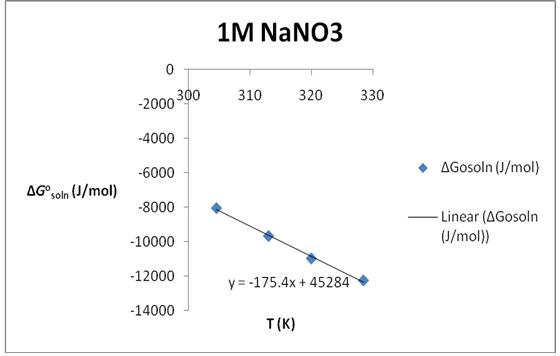
∆S°soln=175.4 J/K*mol; ∆H°soln=45.284 KJ/mol
Table 5: The solubility of KNO3 in 2M NaNO3.
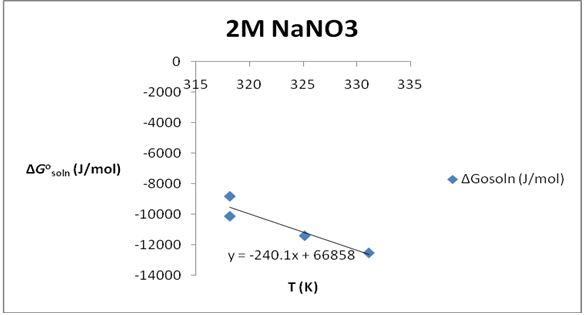
∆S°soln= 240.2 J/K*mol; ∆H°soln=66.858 KJ/mol
Table 6: The solubility of KNO3 in 1M KCl.
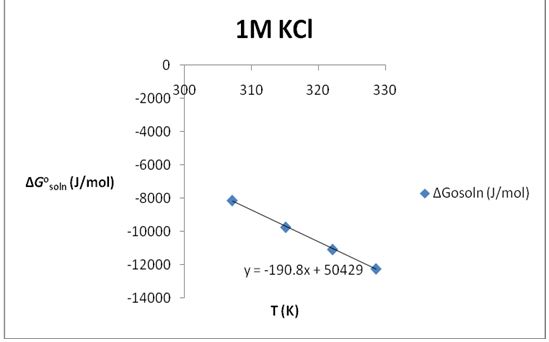
∆S°soln= 190.8 J/K*mol; ∆H°soln=50.429 KJ/mol
Table 7: The solubility of KNO3 in 2M KCl.
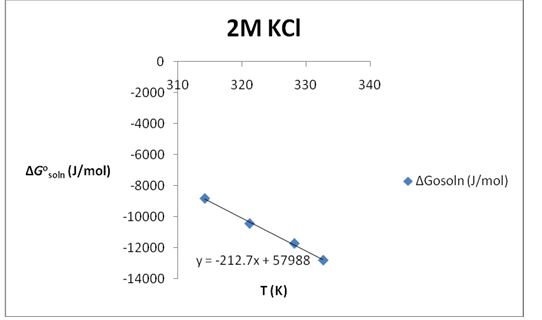
∆S°soln= 212.7 J/K*mol; ∆H°soln=57.988 KJ/mol
Discussion
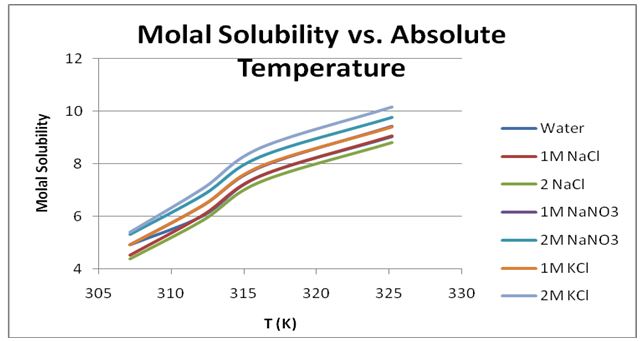
Slight differences were seen in the experimental values and literature values of ∆S°soln and ∆H°soln. The experimental ∆H°soln for KNO3 in water was found to be +56.787 KJ/Mol, whereas the literature value according to Grossie and Underwood was +50.9 KJ/Mol (263). The experimental ∆S°soln, in contrast, was +210.5 J/K*mol while the literature value was +193 J/K*mol. There were slight differences in the molal solubility values from the experiment and the literature values. For example, the experimental molal solubility for KNO3 in water at 20 °C was 4.914, whereas the literature value at the same temperature was 3.13. From figure 9, it was realized that the solubility of KNO3 varied in different solvents.
The lowest solubility was in 2M NaCl, whereas the highest solubility was achieved in 2M KCl. It was realized that the solubility was low in the 1M solutions and higher in the 2M solutions. It was also seen that the solubility in the 1M solutions was lower than in water. However, the highest solubility was seen in a 2M solution of KCl. The differences could be due to experimental errors, such as erroneous thermometer readings as well as taking erroneous weights of solvents and salts. It was also possible that some of the temperature readings were taken too early or too late when determining the solubility of KNO3 in water.
The observed overall trend was that the solubility of KNO3 rose as the temperature was elevated. The observed values were within the experimental uncertainties, such as those reported in other similar experiments. Therefore, it was concluded that the solubility of KNO3 increased with a raise in the temperature of the solution regardless of the nature of the solvent.
Works Cited
Grossie, A David and Kirby Underwood. Laboratory Guide for Chemistry. 6th ed. 2013. Plymouth, MI: Hayden-McNeil. Print.