Introduction
Warfarin (Coumadin) is a prescription drug used as an anticoagulant. Patients under warfarin prescription respond differently to the drug based on their gender, age, related medication, body mass, heredity, and diet as shown by Robert H in the case 3. An overdose or under-dose of warfarin has serious implication, including blood coagulation or excessive bleeding. According to Chua, Abduallh, Yusof, and Gan (2014, p. 4), it is estimated that about 1% and an additional 15% of non-lethal bleeding problems are attributed to warfarin toxicology and warfarin-related deaths respectively. Warfarin was clinically certified as a pharmacological therapy against arterial and venous thromboembolic disease. Nevertheless, multiplicities of warfarin dosage prescription, drugs variety, diseases, improper usages, and nutritional reactions have inhibited the safe application of the drug.
Pharmacodynamic and Pharmacokinetic Implications
Warfarin metabolism entails the inactivation of “s-warfarin by the cytochrome P450 isozyme 2C9 or CYP2C9” (ARUP, 2009). Decreased protein function is witnessed in the CYP2C9*2 type of CYP2C9 gene variant whereas CYP2C9*3 variant exhibits abolished protein function. Warfarin metabolism site is located at vitamin K epoxide reductase (VKOR) region and is influenced by the region’s associate VKORCI gene with the gene’s promoter variant (c -1639 G>A), which is established as a controller of warfarin metabolism gene expression. Subsequently, it minimises cases of warfarin sensitivity by lowering the amount of VKOR. According to Meijer, Kim and Schulman (2010, p. 225) warfarin gene expression includes the intron 1 VKORCI variant c. 173+1000C>T. An estimated 40%-63% variability in warfarin dose therapy is attributed to VKORCI mutation from the combination of the *2 and *3 CYP2C9 variants. Serious complications from unsafe usages of warfarin principally involve antithrombotic effects, which are traced back to the interference of vitamin K synthesis dependent variables II, VII, IX and X by the hepatic circulatory system. Lee et al. (2010, p. 217) attribute antithrombotic effects of warfarin administrations to further interference in the production of protein C and S anticoagulant.
Biological activation of vitamin K clotting factors requires a mandatory gramma-carboxylation of the glutamic acid residues located at the NH2-terminal. Vitamin KH2 enzyme is used in the reaction as a condensed variety of vitamin K. Glutamic acid residue, Gamma-carboxylation yields vitamin KH2 oxide KO, an inert variety of vitamin K. Vitamin KO is then converted to vitamin K by K epoxide reductase (VKOR), and then to KH2 which is catalysed by vitamin K1 reductase. Reuse of vitamin K in the hepatic circulatory system provides incessant supply of vitamin KH2 for clotting variants production.
Warfarin is established as an inhibitor of vitamin K hepatic recycling agents, VKOR, and vitamin K1 reductase. This causes high levels of inert vitamin KO, which limits production of vitamin K associated clotting variants. Nevertheless, overt biologic effects of warfarin are only evident after exhaustion of priory activated clotting variants. Further, such exhaustion is determined by the half-life of each one of the activated clotting variant. Thus, complete anticoagulant effect of warfarin is expected to delay for 3-7 days since the commencement of warfarin therapy or dose adjustment. Disruption of warfarin dose prescription causes build up vitamin K associated clotting variants, before relapse to biologically normal levels (indicated in the case of Robert H).
Monitoring of warfarin requires clinical measurement of the drug’s clinical impacts in the circulatory system. Such clinical investigations involve computation of the time interval required for clotting to occur. Analysis of warfarin clotting with time involves measurement of internationally normalisation ratio (INR) through a protime or prothrombin time (PT). Warfarin therapy is necessary in prevention of cases of pathologic clotting and in reduction of probability of hemorrhagic problems. INR estimates of 2-3 are considered optimal, though higher INRs requirements are recommended during automatic substitution of a valve. Mlynarsky, Bejarano-Achache, Muszkat, and Caraco (2012, p 619) recommend in-depth knowledge and experience in pharmacodynamic and pharmacokinetic factors role in anticoagulation processes to enhance effectiveness and safety of warfarin therapy.
Warfarin dosage for an optimal INR varies among individuals and ranges between 0.5-2 mg with an estimated average of 4-5 mg single daily dose for an anticipated INR of 2-3. Equally, various forms of warfarin initiation and sustenance therapy strategies are available for both elderly and adult patients, which entirely depend on measurement of INR. Initiation of warfarin therapy requires a follow-up measurement of INR build-up within the three-warfarin doses period and until the lower limit of clinically monitored therapeutic dynamics is achieved. Zee and Halperin (2010, p 549) rationalise that warfarin sustenance and adjustment therapy against measurement of variants is associated with changes in INR. In infants, warfarin therapy is done under an authoritatively loading protocol. Infants’ warfarin prescriptions are relatively less compared to adults’ prescription.
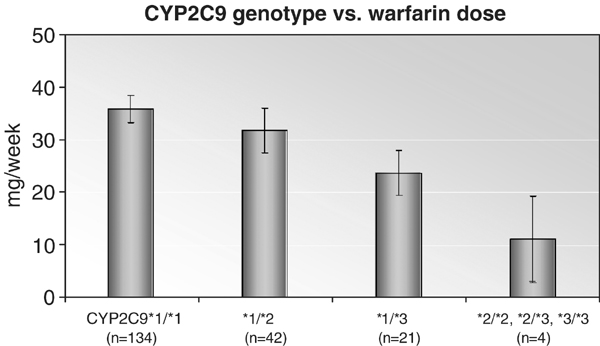
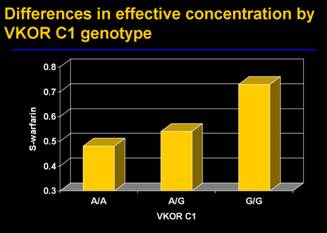
People with either CYP2C9*2 and CYP2C9*3 show dysfunctional s-warfarin metabolism characterised by longer removal of warfarin from their metabolic system because of a protracted warfarin half-life. Warfarin dosage of up to 5 mg/g can lead to serious side effects including overly anticoagulant response and lethal bleeding problems in patients with CYP2C9 and/or VKORCI variants (see case 3 of Robert H.). Patients exhibiting dysfunctional CYP2C9 symptoms take longer to achieve an INR stable condition from the protracted warfarin half-life. Therefore, CYP2C9 factors are employed for a limited INR assessment and dosing reticulation for an enhanced and stable build-up of warfarin.
Disparity in VKORCI is empirically attributed to variations in warfarin resistance and sensitivity. Further disparity in warfarin concentration is attributed to the general promoter mutation (c -1639G>A). Warfarin inhibits vitamin K epoxide reductase (VKOR) associated with the recycling of ‘vitamin K and the enzymatic clot factors II, VII, IX and X” (ARUP, 2009) and it applies anticoagulant effects on clot factor concentration.
The history of a patient’s family responses to warfarin dosing is an important consideration in warfarin therapy prescription. This involves investigation of a possible case of problem related to warfarin dosages in the family. Thus, an appointment with a clinical pharmacist is a necessary requirement for analysis of a patient’s genotype using clinical data. A negative clinical result for mutation case invalidates the possibility of gene triggered warfarin sensitivity. A case of CYP2C9 or VKORCI mutation increases chances of warfarin physiology, longer warfarin half-life, and/or the possibility of increased drug sensitivity.
A combination of polymerase chain reaction and unlabeled probe and melting-curve methodology is applied in the detection of CYP2C9*2 (c430C>T), CYP2C9*3 (c1075A>C), and VKORCI (c -1639G>A). More than 90% of lethal CYP2C9 cases of mutations are clinically diagnosed in Caucasians, whereas others with the same mutation are empirically inexistent in other ethnic or racial communities (Lee et al. 2012, p 276).
In a clinical detection of warfarin sensitivity using CYP2C9*2, CYP2C9*3, VKORCI and/or c -1639G>, mutation detection overlooks other forms of mutations associated with warfarin sensitivity. Equally, other somatic genes and non-genes variants with effects on warfarin sensitivity are not overlooked in clinical detection using s-warfarin metabolism genes. Diagnosis errors are also possible from primer-site mutations. Diagnosis of warfarin metabolism associated genes does not substitute the requirement for drug investigation therapy or other right clinical monitoring interventions.
Conclusion
Warfarin drugs come in both injectable and tablet forms. Thus, they can be administered orally or through injections. Warfarin is pharmaceutically labelled as Coumadin, which is manufactured in a variety of colours to distinguish a particular Coumadin dose strength. Additional dose strength score is also marked on a Coumadin tablet. Dyes are excluded from Coumadin packages due to their allergic effects on patients following warfarin therapy initiation.
Reference List
ARUP 2009, Warfarin Sensitivity (CYP2C9 & VKORC1) 3 Mutations, Web.
Chua, Y A, Abdullah, W Z, Yusof, Z & Gan, S H 2014, ‘A New Nested Allele-Specific Multiplex Polymerase Chain Reaction Method for Haplotyping of VKORC1 Gene to Predict Warfarin Sensitivity’, BioMed Research International, vol. 2014, no. 2014 , pp. 1-6. Web.
Lee, K E, Chang, B C, Kim, H O, Yoon, I K, Lee, N R, Park, H Y & Gwak, H S 2012, ‘Effects of CYP4F2 gene polymorphisms on warfarin clearance and sensitivity in Korean patients with mechanical cardiac valves’, Therapeutic drug monitoring, vol. 34, no. 3, pp. 275-82.
Lee, S, Jang, Y J, Cha, E, Kim, H, Lee, S S & Shin, J 2010, ‘A haplotype of CYP2C9 associated with warfarin sensitivity in mechanical heart valve replacement patients’, British Journal of Clinical Pharmacology, vol. 70, no. 2, pp. 213-221.
Meijer, K, Kim, Y, & Schulman, S 2010, ‘Decreasing warfarin sensitivity during the first three months after heart valve surgery: Implications for dosing’, Thrombosis Research, vol. 125, no. 3, pp. 224-229.
Mlynarsky, L, Bejarano-Achache, I, Muszkat, M & Caraco, Y 2012, ‘Factor VII R353Q genetic polymorphism is associated with altered warfarin sensitivity among CYP2C9 *1/*1 carriers’, European Journal of Clinical Pharmacology, vol. 68, no. 5, PP. 617-627.
Zee, S A & Halperin, J L 2010, ‘Anticoagulant therapy: Warfarin sensitivity genotyping closer to clinical practice’, Nature Reviews Cardiology, vol. 7, no. 10, PP. 549-550.