Introduction
This is a comprehensive essay which outlines the process of tablet manufacture with a detailed discussion on aspirin. Background information is given on tablet manufacture in the ancient days. This essay will bring to light the whole process of pharmaceutical manufacture of tablet making the description as detailed as possible.
History of Tablets
The use of tablet as a means of drug administration to the body is not a recent practice. It has been shown beyond doubt that tablet were used by the ancient Greeks. It is quite amazing that the tablets used by these ancient people are very similar to what is currently used in the modern day life; think in terms of stability and the different constituents in the tablets. The acknowledgement that the ancient Greeks used drugs in tablet forms surfaced upon the discovery of an ancient shipwreck where 2,000 year old drugs were found intact. Papapostolou (2010) reported that a ship which sank off the coast of Italy n 130 BC gave valuable revelation about the knowledge that the ancient Greeks possessed on matters of drugs. It was reported that tablets were recovered from the wrecks of the ship and some DNA analysis was carried out on these drugs showing amazing knowledge which was not expected to be in existence then.
According to the laboratory tests carried out on these tablets it was shown that each single tablet of them contained a variety of plant extracts which were reported to be more than 10. The art of mixing different extracts in a tablet is in line with modern day manufacture of tablets. The fact that the tablets were still intact by the time they were discovered is a sure proof that the ancient physicians knew much about tablet stability. The laboratory analysis showed the following extracts “carrot, radish, celery, wild onion, oak, cabbage, alfalfa and yarrow” (Papapostolou 2010, 1) most of which have been claimed to have high medicinal value:
Yarrow staunched the flow of blood from wounds, and Pedanius Dioscorides, a physician and pharmacologist in Rome in the first century AD, described the carrot as a panacea for a number of problems. “They say that reptiles do not harm people who have taken it in advance; it also aids conception,” he wrote around 60 AD. (Papapostolou 2010, P. 1)
This discovery of the ancient Greece pills have shown that the arguments made by Dioscorides and Galen of Pergamon were not just about quackery drugs as there are possibilities that the extracts in these tablets may treat some illness effectively. Studies are still taking place to resolve the drug therian which has been described by Galen and claimed to have more than eighty plant extracts (Papapostolou 2010, p. 1). It has not been shown yet how the ancient Greeks went about the process of measuring and mixing the different plant extracts but it is evident that they had rich knowledge about the tablet formation.
Tablet Formation
Generally the process of tablet formation can be said to go through broad stages which are communition, mixing and compression. Simply defined communition is the process of reducing large sized solids into small sizes. The process is manly mechanical and is used in the process of tablet manufacture. After crushing different extracts they are then mixed and compacted later to form tablet. The process of making a tablet has many cross checks to ensure high accuracy is maintained.
Weighing Up
The very first stage in the process of tablet manufacture is the weighing up exercise whereby the active and inactive constituents of the drug required are accurately measured as per the drug formula. Whether the tablets are to be manufactured on a large or small scale the measuring has to follow the Standard Operating Procedure for each measurement of each part. As an internal check, two people work on the same weight with one weighing and the second verifying the measured weight (Woodward 2011, p. 1). The weighing exercise is followed by granulation stage.
Granulation
At the granulation stage, the constituents are crushed together again and again to form fine powder which can be compressed to form the tablets. The granulation process used varies from drug to drug however three methods have pointed out: dry granulation, wet granulation and direct compression.
Dry Granulation
This type is used to manufacture aspirin tablets. Dry granulation is applied for those drugs which are highly sensitive to moisture and heat and is appropriate for drugs with cohesive powers (Dieing, Hollricher and Toporski 2011, p. 191). The active part of the aspirin tablet is the corn starch. The granulation of aspirin is described as follows:
The corn starch is dispensed into cold purified water, then heated and stirred until a translucent paste forms. The corn starch, the active ingredient, and part of the lubricant are next poured into one sterile canister, and the canister is wheeled to a mixing machine called a Glen Mixer. Mixing blends the ingredients as well as expels air from the mixture. (Ling 2011, p. 1)
By mechanical means the mixture is separated into units referred to as slugs. The slugs are then pushed through a mesh screen to form small uniform granules. A Fitzpatrickmill is used for the case of large scale production of tablets. A lubricant is added to these uniform granules and they are ready for the compression stage (Ling 2011, p. 1).
Moist or Wet Granulation
This involves the use of water in the blending process. This granulation type is used in the manufacture of sleeping pills. The constituents of the drugs are put in a blender and some water added to the ingredients. The wetted ingredients are mixed and form a thick paste. The thick paste is then subjected to some means of crushing for example in the case of sleeping pills a hopper with rotating hammers is used to grind the paste. The next step is carried as follows “resultant thick paste is then dried and then pressed through a screen which has small uniform sized holes to create granules of the same size” (Woodward 2011, p. 1). The granules coming out of the screen are ready for compression to form tablets (Woodward 2011, p. 1).
Direct Compression
This method is used for substances that exhibit cohesive qualities. The direct compression method omits the use of the granulation stage because the substances which are directly compressed are already granules. Amjad (2005) described the granules used in direct compression as thus: “they are small crystallite randomly embedded in a matrix of some amorphous material which readily imparts the desired characteristics of consolidation including plastically deformation to relieve internal stress and strong bonding surface” (306).
Tablet Presses
After the process of granulation as explained above in section 3.2, the granules are lubricated to ensure that they do not stick to walls of the tablet presses when they are being pressed. The lubricants used are discussed in a different section below. Tablet presses are simple to complicated machines which are used to press the thick paste to form tablets. The tablet presses range from those which can produce one tablet at a time to those which can produce thousands of tablets at the same time. Presses are designed to ensure high accuracy is maintained in the formation of the tablet.
Single Tablet Presses
Single tablet presses do exist and are used for manufacturing of tablets on small scale. A good example of such a press is the C&C600 model. C&C600 press machine produces single tablets continuously and is specifically suited for “pharmacy, chemical industry, electrical industry, food, laboratory which needs to make powder and granular raw material into tablets” (Cambcavi 2006, p. 1). The design of the machine should be in such a way that it protects the thick paste and tablet formed from dust pollution.
Multi Tablet Presses
Presses which produce many tablets at the same time are used by pharmaceutical industries for purposes of large scale release. Most of these presses are designed to compress almost all types of pastes into tablets. Some models such as the C&C800A are designed to press paste into “circular tablet, abnormal shaped tablet, graphical tablet, double sided letter tablet, and even redesign the tablet to cope with the user’s need” (Cambcavi 2006, p. 1).
The Pressing Process
The pressing process depends on the machine used. The pressing process for sleeping drugs is described as thus:
Workers feed the powder into hoppers above a tabletting machine. This machine has a rotating tray of hollow dies in the shape of half the finished tablet. The powder falls from the hopper into the hollow dies. Then an arm holding an inverted die of the same shape descends and exerts several tons of pressure on the powder. This compresses the powder into tablet form. Depending on the size of the tabletting machine, it may be able to produce thousands of tablets a minute. The tablets fall onto a belt that counts them into sterile packaging. The packages are sealed, and readied for shipping. (Woodward 2011, p. 1)
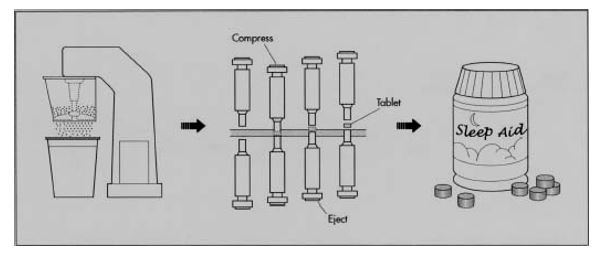
Single Tablet Pressing
Many of the single tablet tablets presses are electrically operated though some which are operated mechanically are available. The single tablet presses usually have the paste fed into one tablet mold by use of a feed shoe. The process can be explained as follows:
The feed shoe passes over the dye cavity and releases the mixture. The feed shoe then retracts and scrapes all excess mixture away from the dye cavity. A punch—a short steel rod—the size of the dye cavity descends into the dye, compressing the mixture into a tablet. The punch then retracts, while a punch below the dye cavity rises into the cavity and ejects the tablet. As the feed shoe returns to fill the dye cavity again, it pushes the compressed tablet from the dye platform. (Ling 2011, p.1)
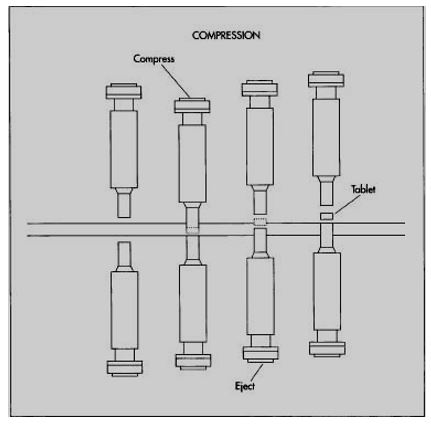
Multi Tablet Pressing
For the case of multi tablet pressing the process involves the use of multiple dye cavities situated on a large steel plate. The process is as follows:
The plate revolves as the mixture is dispensed through the feed line, rapidly filling each dye cavity. Punches, both above and below the dye cavities, rotate in sequence with the rotation of the dye cavities. Rollers on top of the upper punches press the punches down onto the dye cavities, compressing the mixture into tablets, while roller-activated punches beneath the dye cavities lift up and eject the tablets from the dye platform. (Ling 2011, p. 1)
Excipients of Tablet
In the process of tablet formation there are components which are added to improve the qualities of the tablet. The components added are referred to as excipients and are meant to “improve physical appearance, enhance stability and facilitate disintegration process following administration” (Amjad 1997, p. 304). The most common excipients are “diluents (or fillers), binders, anti-adherents, glidants, lubricants, colorants, and disintegrating agents” (Amjad 1997, 304). It has been noted that the excipients are non therapeutic in nature but play significant roles for instance in enhancing the release of the drug an significant factor in the determination of the clinical effect of a drug. Care should be taken when blending drugs with excipicients not to negatively the quality and efficacy of a drug. The sections below briefly explain expicients.
Diluents
Diluents form bulky of the tablet and as such are also referred to as fillers. The fillers make the drug easier to handle (Winfield and Richards 2004, p. 230). Choosing of diluents for a drug needs to be done carefully especially for drugs that are moisture sensitive. As a general rule diluents with bound water are given preference over the unbound water ones. The diluents have different behaviours when subjected to compression forces implying that this is likely to affect the following significant characters of a drug: “the disintegration, dissolution and biopharmaceutical profile” (Amjad 1997, p. 307). Some of the commonly used compounds as diluents include “lactose, starch, mannitol, sorbitol, microcrystalline cellulose, calcium phosphate-trihydrate and calcium sulphate-dihydrate” (Amjad 1997, p. 307).
Binders
These are compounds which are added to the drug powder to enhance the cohesiveness of the powder thus making it possible for the granulation process to be easy. Two factors taken into consideration when choosing binders are compatibility and cohesiveness power. Care has to be taken on the amount of binder added to the tablet as too little will make a loose table and too much of the binder will cause a dissolution problem for drug (Remington 2005, p. 1040). Zimmerman (2006) has given a list of excellent binders:
Exemplary binders include acacia, tragacanth, gelatine, starch, cellulose materials such as methyl cellulose and sodium carboxy methyl cellulose, alginic acids and salts thereof, polythene glycol, guar gum, polysaccharide, bentonites, sugars, invert sugars, poloxamers, collagen, albumin, gelatine, cellulosics in nonaqueous solvents, combination thereof and the like. Other binders include, for example, polypropylene glycol, polyxyethylene-polypropylene copolymer, polyethylene ester, polythlene sorbitan ester, polyethylene oxide, combination thereof and other materials known to one of the ordinary skill in the art. (Zimmerman 110)
Disintegrants
These are compounds which are used to increase the ability of a tablet to disintegrate upon being introduced in an aqueous media. Care should be taken to ensure that the correct amount of the disintegrant is added to avoid maldisintegration. If the disintegrant is too much there is a likelihood that the tablet will disintegrate upon being exposed to slight moisture contents but again if the disintegrant is too little then likely the tablet will mostly not disintegrate as expected upon being taken oraly and this will affect the therapeutic value of the tablet (Remington 2005, p. 1040). Examples of disintegrants are: “pregelatininized starch, microcrystalline cellulose, sodium bicarbonate in combination with citric or tartaric acids, alginic acid, and Ion exchange resins” (Carter 2006, p. 1). However it is worth noting that the above mentioned are not very commonly due to the introduction of super disintegrants. Super disintegrants are claimed to be very effective as they enhance the dissolution process faster than the early disintegrants. Super disintegrants swell up many times their original sizes when placed in water as compared to the normal disintegrants. Examples of super disintegrants are:
Modified starches-sodium Carboxymethyl starch (chemically treated potato starch) i.e. sodium starch glycolate (explotab, primogel); Cross-linked polyvinlypyrrolidone-water insoluble and strongly hydrophilic; modified cellulose-internally cross linked form of sodium carboxymethyl cellulose. (Carter 2006, p. 1)
Lubricants, Glidants and Antiadherents
According to Winfield and Richards (2004), lubricants, glidants and antiadherents are “essential for flow of the tablet material into the tablet dies and preventing sticking of the compressed tablet in the punch and die” (230). Magnesium stearate and Talc have been viewed as effective antiadherents while colloidal silica is a preferred glidant. Lubricants are substances which are applied on the powder which is ready for pressing to tablet form. The lubricants are used to make the powder avoid sticking on the sides of the pressing machine after being compressed. Substances which are used as lubricants are usually hydrophilic in nature and include such substances as magnesium, stearic acid, or calcium stearate. Care should be taken on the amount of lubricant used as too little of the lubricant will lead to unsatisfactorily drugs being formed while too much of it will make a tablet which may not disintegrate and dissolve easily (Remington 2005, p. 1040; Wen and Park 2010, p. 106).
Tablet Preservative
The use of preservative is debated. Arguments have been made that by including preservatives in drugs the tablets will be protected in case they get damp especially in the tropical and humid regions. However these arguments have been met with a lot of opposition due to the fact that a tablet getting damp will imply its inherent destruction. Suggestions have been aired advocating for the use of water resistant coating on the drugs to reduce the probability of water being taken in by the drug (Ash and Ash 2004, p. 547).
Colours, Sweeteners and Flavours
Colours and flavours are mostly added to tablets which are meant to be chewed. The colours and flavours are added on the coating of the drugs. Apart from the tablet coating containing some sugars and colours they also protect the drug from damage. Flavours are used when the drug has an unpleasant taste. However it has been pointed out that the enteric coatings enhance the resistance of the tablet to dissolve in the stomach but make it possible for it to be absorbed in the intestine (Winfield and Richards 2004, p. 231).
Dissolution of a Drug
The process of drug dissolution is a very significant one as it ensures that the drug is actually absorbed and consequently utilized by the body. Tablets have to be designed in a way that upon getting in contact with water they disintegrate.
Dissolution Path
The following figure is a simple outline of the disintegration process.
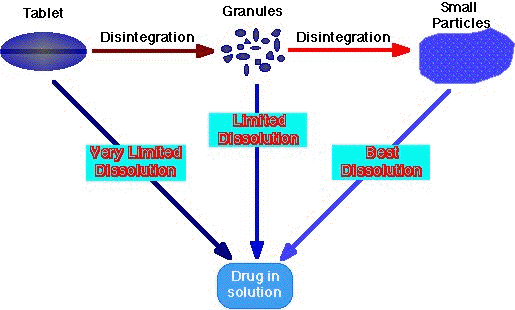
As seen in the above figure, disintegration of a drug is very significant to ensure that the drug wholly dissolved. Intuitively any factor which assists in breaking up the size of the tablet helps in dissolving the drug and thus the drug release.
Factors affecting the Drug Release
As noted in section 4.3 disintegrants are very important in ensuring that drug release takes place once the drug is in touch with the water in the body. Carter (2009) has identified three major mechanisms through which drugs are released to the body: swelling, porosity & capillary action, and deformation (p. 1).
Swelling
Swelling has been claimed to be the major mechanism through which drugs are released. Most of the disintegrants take up water and swell up resulting to reduction of the adhesiveness of the ingredients in the tablet. When the adhesiveness of the constituent parts of the drugs is overcome then they fall apart and are absorbed to the body. A good example of a disintegrant that breaks up a drug by swelling after taking up water is starch.
Porosity and Capillary Action
Some disintergrants do not swell up in the way starch does as seen in section 5.2.1 above but instead they facilitate the disintegration process through porosity action. Disintegrants which do not swell up instead provide path ways through which water is absorbed to break “the interparticulate bonds causing the tablet to break apart” (Carter 2009, p. 1).
Deformation
It has been argued that disintegrants like starch have grains which are said to be elastic naturally implying that even after being deformed they still can regain their shapes. In the case of tableting, the starch used has been claimed to more be permanently deformed and as such it is claimed to be energy abundant. The energy of the starch in the tablet in the compression state is released once the tablet gets in touch with water. Carter (2009) argued that starch which is rich in energy that is one which is in a compressed state has a higher probability of swelling up as compared to the one which is not compressed. Therefore deforming disintegrants which are elastic in nature makes it possible for them to swell up once in contact with water thus disintegrating the tablet.
It has been noted that when corn starch is used it has to be in the range of 5 to 10 percent. It is argued that below the five percent range the compressed starch will not provide enough paths for wicking and thus this will not facilitate the swelling up of the drug. If the range is above ten percent it becomes hard for the tablet to be compressed to the sufficient hardness due to the incompressibility of starch (Carter 2009, p. 1). Other factors which have been listed to affect dissolution of tablet are: “Type and concentration of active ingredient, binder, fillers, and lubricant used, the dissolution testing applied and the tablet manufacturing process” (Carter 2009, p. 1).
Stability of a Tablet
According to Atia (n.d.), stability of a drug is “is the capacity of a drug product to remain within specifications established to ensure its identity, strength quality and purity” (3). There should be an attempt to maintain the stability of a drug as its instability has the following consequences: a drug may not perform as expected due to the effect likely to occur on dissolution and bioavailability of the tablet; and there is a likelihood of substantial changes on the physical appearance of the drug (Atia n.d. p. 3).
The stability of a drug can be classified into three major types: physical, chemical and microbiological stability. When a tablet is physically stable the implication is that its formulation has not been altered in any way. Physical stability is significant because it assures “pharmaceutical elegance, drug content uniformity and drug release rate” (Atia n.d. p. 6). According to Dr. Atia (n.d.), “Microbiological stability implies that the formulation has not suffered from any microbiological attack and is meeting the standards with respect to lack of contamination/sterility” (Ati nd, p. 32) Chemical stability has been viewed as “the lack of any decomposition in the chemical in the chemical moiety that is incorporated in the formulation as the drug, preservatives or any other expicients” (Atia n.d., p. 15). Chemical decomposition of a tablet may have an effect on both the physical and chemical stability of a tablet.
Mechanisms of Degradation
The mechanisms of tablet degradation are hydrolysis, oxidation, and photolysis
Hydrolysis
Hydrolysis of tablet is splitting of a tablet by means of water. This most occurs to tablets which have functional groups which hydrolyse in the presence of water for example aspirin has a functional group ester which is easily hydrolysed by water.
Table 1
Hydrolysis of Aspirin
As seen above in table 1, aspirin has the functional group ester which is easily hydrolysed to form salicyclic acid and acetic acid.
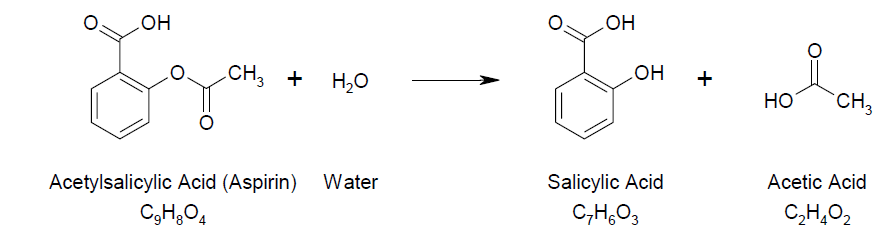
As seen in the above chemical reaction 1, aspirin contains an ester group which is split by water resulting to salicylic acid and acetic acid.
Oxidation and Photolysis
Some drugs are easily oxidised while some are easily degraded by light. Though tablets are affected by oxidation the effect is not as much as in the case of hydrolysis.
Eliminating the degradation effect
Care ought to be taken to protect tablets from degradation. To reduce the effect of degradation on drugs, packaging and coating of drugs ought to be carried out carefully to ensure that the tablets are protected from the degrading effect.
Tablet Coating
Coating of tablets serves many purposes. The coats can be used as water resistant, sweeteners, and even for flavouring purpose. Coats which are water resistant are good as they help to reduce the degradation effect resulting from hydrolysis process and oxidation. Coating of aspirin has been associated more with problems it causes in the stomach (read section 7.1 below) than trying to reduce degradation of the drug.
Tablet Package
Tablet packaging is very significant and should be done with utmost care. The package used should be water resistant. Aspirin has been shown to be stable in dry air but readily hydrolyses in moist conditions and therefore should be “stored in tight, moisture resistant containers” (Mikota 2006, p. 1).
Aspirin
Aspirin is a popular drug and used on a non-prescription basis. It is used to “relieve pain (analgesic), to prevent and reduce fever (antipyretic), to reduce inflammation, (a nonsteroidal anti-inflammatory drug, NSAID), and as a prophylactic to prevent myocardial infarction and stroke for people at risk” (Dashman and Blocker 2005, p. 215).
History of Aspirin
Felix Hoffman played an important role in the improvement of aspirin for human consumption. In 1890 Hoffman managed to make the drug less irritating by modifying the salicylic acid in it to be less acidic. This is was one of the many modifications through which the bark of the willow trees had undergone. Bellis (2011) has claimed that as early as between 466 B.C and 377 B.C it was known that the willow tree contained some substance which could relief pain. It is claimed that Hippocrates left some records indicating “pain relief treatments, including the use of powder made from the bark and leaves of the willow tree to help heal headaches, pains and fevers” (Bellis 2011, P. 1).
In 1828 it was discovered that the willow tree contained the compound salicin which was responsible for the pain relieving. It is recorded that early in 1826 Brugnatelli and Fontana had extracted salicin but in a very impure state. Johann Buchner in 1828 had extracted crystals of salicin. In 1829 Henri Leroux developed a better extraction method which gave more yield of salicin. In 1838 Raffaele Piria made some significant improvement by “splitting salicin into a sugar and an aromatic component (salicylaldehyde) and converted the latter, by hydrolysis and oxidation, to an acid of crystallised colourless needles, which he named salicylic acid” (Bellis 2011, p. 1).
Salicylic acid is tough on stomach and so the remaining challenge was how to buffer the acid (Reid, McKay, and Walters, 2010, p. 246; Wachter n.d., p. 1). Charles Frederic Gerhardt by using sodium was able to buffer the acid in 1853. It is recorded that though what Charles had discovered worked he did not pursue it for marketing purpose and thus its formulae was forgotten until its rediscovery by Felix Hoffman later in 1899. Hoffman was working for Bayer and the company started producing the drug. It is said that the people at Bayer named the drug aspirin and patented it in 1900 (Pavia 2005, p. 55; Bellis 2011, p. 1). At first the drug was sold as a powder but in 1915 the first tablets were manufactured (Bellis 2011, p. 1).
Pharmacokinetics of Aspirin
The process of aspirin hydrolysis in the stomach is the same as shown in reaction 1 above in section 6.1.1.1. It has been shown that aspirin is absorbed from the stomach at a very high rate. How fast aspirin is absorbed has been shown to be a function of the following factors “stomach content, gastric emptying times, tablet disintegration rates and gastric pH” (Mikota 2006, p. 1).
The process of hydrolysis of aspirin makes it possible for it to be absorbed and spread out in the body (King and Brucker, 2009, p. 317; Tozer and Rowland 2008, p. 1). It has been shown that the highest levels of hydrolysed aspirin are found in the following areas of the body “liver, heart, lungs, renal cortex, and plasma” (Mikota 2006, p. 1). The following facts have been observed in relation to aspirin hydrolysis:
The amount of plasma protein binding is variable, depending on species, serum salicylate and albumin concentrations. At lower salicylate concentrations, it is 90% protein bound, but only 70% protein bound at higher concentrations. Salicylate is excreted into milk, but levels appear to be very low. Salicylate will cross the placenta, and fetal levels may actually exceed those found in the mother. (Mikota 2006, p. 1)
It has been shown that the metabolism of Salicylate in carried out through conjugation with glucuronic acid through glucoronyl transferase. When the drugs is used on cats it tends to have a prolonged half life because of the lack of glucoronyl transferase in cats thus the enzymatic pathway is omitted (Siddik 2009, p. 129; Fraise, Lambert and Maillard 2008, p. 1). There are minor metabolites which have been reported to be formed: “gentisic acid and 2, 3-dihydroxybenzoic acid, and 2, 3, 5-trihydroxybenzoic acid” (Mikota 2006, p. 1). Though gentisic acid has been said to be an active component it is doubted whether it plays any significant part in the therapeutic process because of its availability in small concentrations. It has been shown that
The rate of metabolism is determined by both first order kinetics and dose-dependent kinetics depending on which metabolic pathway is looked at. Generally, steady-state serum levels will increase to levels higher (proportionally) than expected with dosage increases. These effects have not been well studied in domestic animals, however. (Mikota 2006, p. 1)
The method of excretion of salicylate is by means of the kidneys through filitration and renal tubular secretion. It has been observed that salicylate is easily excreted when the pH is high. Peritoneal dialysis is used to excrete the metabolites and hemodialysis even makes the whole process faster.
Spectrophotometry of Aspirin
Spectrophotometry is used to measure the levels of serum salicylate. This method is preffered because it reduces the limits the detection to around 100micrograms/ml; this amount has been claimed to be enough for toxic and therapeutic reasons (Schror 2009, p. 30).
Conclusion
It has been shown that tablets were used by the ancient Greeks. The tablets contained many extracts from many plants which have been revealed to be medicinal. The tablet discovered from a shipwreck showed the huge knowledge that the Greeks possessed about tablet manufacture. The stability of the pills which were discovered was also quite amazing. Modern day tablet manufacture goes through three broad stages which involve communition, mixing and comprehension. The process of tablet manufacture has many cross checks to ensure that an error is not committed which will affect the performance of the tablet.
In the process of drug formation among the constituents that are added in the tablet are excipients. Excipients serve many purposes and enhance the performance of the drug. Care ought to be taken to ensure that the excipients used do not negatively affect the performance of the tablet; it has been not noted that a failure to use the correct amount of a given excipicient in a drug then there will be likelihood that the tablet performance will be affected negatively. Excipicients are added in form of diluents, binders, disintegranst, lubricants, glidants, antiadherents, colours, sweeteners and flavours. Three majors way in which have been identified through which tablets are released to the body are: swelling, porosity & capillary action, and deformation. Tablets are degraded through three means: hydrolysis, oxidation, and photolysis. Hydrolysis occurs to tablets which have functional groups which hydrolyse in the presence of water for example aspirin has a functional group ester which is easily hydrolysed by water. Tablets can also be degraded through oxidation and photolysis. Degrading can be reduced through packaging and coating of tablets.
A good example of a tablet is aspirin. Aspirin has been in use almost through the history of humanity. Though it was shown to have been known as early as 377 B.C., it was until 1890 that it consumption started to spread out widely. Aspiring heals through its hydrolysis in the body to form salicylic acid which is said to have the therapeutic value.
Bibliography
Amjad, Z. (1997) Calcium phosphates in biological and industrial systems. New York, Springer.
Ash, M and Ash, I. (2004) Handbook of preservatives. New York, Synapse Info Resources.
Atia, G. (n.d.) Stability of Drugs. Pharmaceutics. Web.
Bellis, M. (2011). History of Aspirin. Inventors. Web.
Bourne, D. (2001) Tablet Dissolution. Boomer. Web.
Cambcavi. (n.d.) Rotating Tablet Press Machine. CAMBCAVI. Web.
Carter, J. (2006) The Role Of Disintegrants In Solid Oral Dosage Manufacturing. [Online] Carter Pharmaceutical Consulting Inc. Web.
Dashman, T and Blocker, D. (2005) Laboratory Manual for Human New York, Taylor & Francis.
Dieing, T., Hollricher, O and Toporski, J. (2011) Confocal Raman Microscopy. New York, Springer.
Fraise, A., Lambert, P., and Maillard, J. (2008) Russell, Hugo & Ayliffe’s Principles and Practice of Disinfection, Preservation & Sterilization. New York, Wiley-Blackwell.
King, T and Brucker, M. (2009) Pharmacology for Women’s Health New York, Jones & Bartlett Learning.
Ling, G. (n.d.) Aspirin. How Products are made. Web.
Mikota, S. (2006) Aspirin. The Elephant Formulary. Web.
Papapostolou, A. (2010) 2,000 Year Old Medicinal Pills Found in Ancient Greek Shipwreck. Greek Reporter. Web.
Pavia, D. (2005) Introduction to organic laboratory techniques: a small scale approach. New York, Cengage Learning.
Reid, J., McKay, G., and Walters, M. (2010) Lecture Notes: Clinical Pharmacology and Therapeutics New York, John Wiley and Sons.
Remington. (2005) Remington: the science and practice of pharmacy. New York, Lippincott Williams & Wilkins.
Schror, K. (2009) Acetylsalicylic acid. New York, Wiley-VCH.
Siddik, Z. (2009) Checkpoint Controls and Targets in Cancer Therapy. New York, Springer.
Tozer, T and Rowland, M. (2006) Introduction to pharmacokinetics and pharmacodynamics: the quantitative basis of drug. New York, Williams & Wilkins.
Wachter, N. (n.d.) Acetylsalicylic Acid. Chemistry Explained. Web.
Wen, H and Park, K. (2010) Oral Controlled Release Formulation Design and Drug Delivery: Theory to Practice. New York, John Wiley and Sons.
Winfield, A and Richards, M. (2004) Pharmaceutical practice. New York, Elsevier Health Sciences.
Woodward, A. (n.d.) Sleeping Pill. How Products are made. Web.
Zimmerman, F. (2006) Hypochondriac’s Guide to Tamiflu (Oseltamvir) for Bird Flu: Public Information Resources. New York, Nimble Books LLC.