Water is an essential resource that cycle through the environment (ACSSU222). Water acts directly as the source of life for plants because it carries nutrients, from which plants derive their food. When it comes to animals, water is an essential aspect of animals’ life. Animals usually feed on plants, which in turn depend on water for growth and survival. Thus, water also acts as a source of life for animals. In addition to that, water provides a natural habitat for many animal and plant species, therefore ensuring their survival. The phrase “water cycle” refers to the continuous movement of water from the surface of the Earth to the atmosphere, and then back to the surface of the Earth (Nelson, 2002). Nelson (2002) asserts that during this cycle, water moves typically from the Earth’s surface and the surface of water bodies, such as oceans and lakes, into the atmosphere. Here, it is cooled down and falls back to the surface of the Earth.
Natural and processed materials have a range of physical properties; these properties can influence their use (ACSSU074). Natural and processed materials can either be categorized as solids, liquids or gases according to their physical properties. Solids, liquids and gases have different observable properties and behave in different ways (ACSSU077). Solids are compact, and they have definite shape and volume. The reason why solids are dense is that the molecules that make up the substance are close to one another; thus, there is no space between the molecules (Nelson, 2002).
On the other hand, liquids do not have a definite shape but instead they adopt the shapes of the vessel containing them, however, just like solids, fluids also possess definite volume (Nelson, 2002). The molecules that make up liquids are loosely packed, allowing for big spaces to exist between the molecules. This is because the molecules in gases are more loosely packed, allowing for even bigger intermolecular spaces. Gases adapt the volume of the container enclosing them (Nelson, 2002).
A change of state between solid and liquid can be caused by adding or removing heat (ACSSU046). Removal of heat from a substance in the liquid state will result to the substance changing into solid whereas addition of heat from a substance in solid-state will result in the substance shifting states from solid to a liquid state. The change of state is brought about by the fact that addition of heat increases the kinetic energy of the molecules thus increasing their ability to move and be further away from one another and that is why the addition of heat results in a substance shifting states from solid to liquid (Olien, 2005). On the other hand, removal of heat reduces the kinetic energy of the molecules resulting in less movement of the same. Consequently, the molecules are tightly packed together, and thus the resultant state is solid (Olien, 2005).
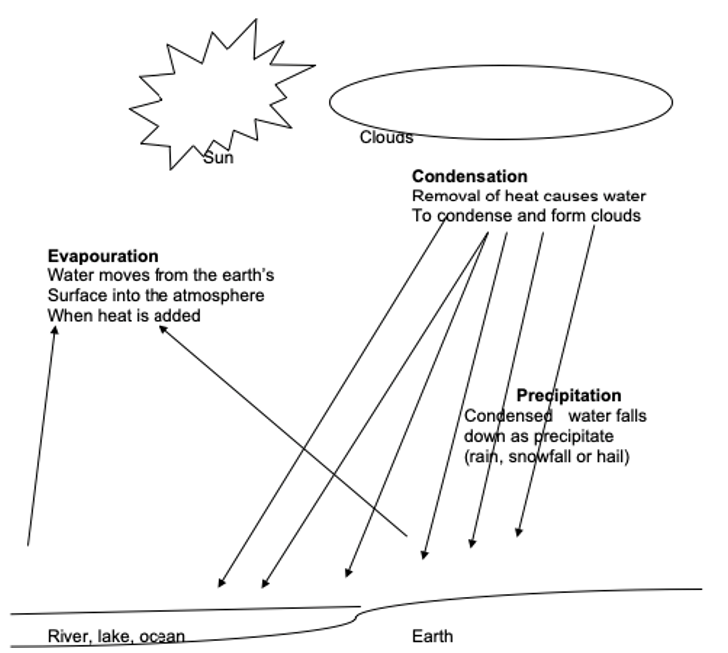
Description of the Diagram
The diagram is a representation of the water cycle. It shows the processes involved in the cycle: condensation, precipitation, and evaporation. During evaporation, waters move from the Earth’s surface to the atmosphere, a process that involves the addition of heat. During condensation, heat is removed from the water, which results in cloud formation. During precipitation, water condenses and falls to the ground as hail, snowfall, or rain.
Evaporation
According to Olien (2005), the processes involved in facilitating the water cycle are evaporation, condensation, and precipitation. Evaporation is the process by which liquids are transformed into a vapour state and rises to the atmosphere (Koontz, 2011). This, therefore, implies that during evaporation, water changes state from liquid to vapour. According to Koontz (2011), this change of state happens when the heat is added to the water. The heat that causes water typically to evaporate from the Earth’s surface comes from the sun. Evaporation takes place from various places on the Earth’s surface, including lakes, oceans, and rivers. In addition to the three places mentioned, water also evaporates from the plants through a process known as transpiration. Transpiration involves the loss of water by plants through various surfaces on their bodies, such as the leaves and stems, and this water finds its way into the atmosphere (Koontz, 2011).
Condensation
In addition to evaporation, the second process that is involved in the water cycle is called condensation. Condensation is the process by which water vapour turns into liquid (Petersen, Sack & Gabler, 2011). The water vapour that evaporates from the surface of the Earth rises to the atmosphere, and as a result of a fall in temperature, the vapour turns (condenses) into liquid. Condensation, therefore, involves the subtraction of heat from a substance, whereby if a substance in a gaseous state is subjected to low temperatures, it turns back to a liquid state.
Precipitation
The third process involved in the water cycle is precipitation. According to Petersen, Sack & Gabler (2011), precipitation occurs as a result of the accumulation of the condensed water vapour in the atmosphere. Petersen, Sack & Gabler (2011) explains that the condensed water vapour usually accumulates and forms clouds. After some time, the amount of accumulated clouds gets too heavy to be suspended in the atmosphere. Consequently, the cloud, which is an accumulation of droplets of water, falls back to the Earth’s surface in the form of rainfall, hail, or snowfall.
Changes to materials can be reversible, such as melting, freezing, evaporating; or irreversible, such as burning and rusting (ACSSU095). Reversible changes are those changes whereby various processes can be applied to the material to enable it to go back to its previous state (Petersen, Sack & Gabler, 2011). These processes usually involve either addition or removal of heat. For instance, a process such evaporation is reversible because evaporation facilitates the changing of state from liquid to the gaseous state through the addition of heat. However, when the heat is removed from the gaseous state, the substance will revert to a liquid state, and therefore such a change is reversible. Just like evaporation, condensation and precipitation are also reversible as in both the two, the substance can be converted to its previous state. However, there are also those changes that are referred to as irreversible changes. This is because once a substance has undergone these changes, no process will be able to revert it to its original state and such changes are rusting and burning (Petersen, Sack & Gabler, 2011).
Environmental Factors influencing the water cycle
Several environmental factors influence the water cycle. It is important to know and understand these factors in order to grasp the concept behind the water cycle. First and foremost, environmental temperature is a very important factor, as far as the water cycle is concerned. As already stated, the various processes that are involved in the water cycle are dependent upon temperature variations. This consists of either addition or subtraction of temperature from water mass. For this reason, the temperature is an important determinant when it comes to determining the course of the water cycle.
High temperatures will result in a high evaporation rate since the amount of heat being added to water will be high (Smithson, Addison & Atkinson, 2002). On the other hand, low temperatures will result in a high condensation rate, because water will be losing heat at a fast pace. Consequently, low temperatures will also increase precipitation. This explains why there is a lot of snowfall during winter; the temperatures at this time are low. Other factors, such as the presence of water bodies and vegetation, will also increase the amount of water that can evaporate into the atmosphere. This will also increase the precipitation rate (Australian Curriculum, 2013).
Impact of Human Activities
Having seen the environmental influence on water, it is now easy to understand how human activity can impact this cycle. Human activities that directly increase environmental temperature will result in a high evaporation rate. Consequently, a lot of moisture will be present in the atmosphere. In addition to that, the destruction of vegetation and water bodies by humans can also greatly influence the water cycle, whereby such activity will result in less water evaporating to the atmosphere (Rice, 2007). Consequently, the amount of water that condenses to form clouds will be reduced, thereby reducing the rate of precipitation.
References
Australian Curriculum. (2013). Science. Web.
Koontz, R. M. (2011). Water Goes Round: The Water Cycle. Michigan: Capstone.
Nelson, R. (2002). The Water Cycle. Sydney: Lerner Publications.
Olien, R. (2005). The Water Cycle. Michigan: Capstone.
Petersen, J. F., Sack, D. I. & Gabler, R. E. (2011). Physical Geography. New York: Cengage Learning.
Rice, W. B. (2007). Inside the Water Cycle: Earth and Space Science (Science Readers). London: Taylor & Francis.
Smithson, P., Addison, K., & Atkinson, K. (2002). Fundamentals of Physical Environment. London: Routledge.