Introduction
West Nile Virus (WNV) is an infectious virus that resides in birds as native reservoir hosts and infects humans, horses, and other mammals via mosquitoes, which act as vectors. Given that WNV infects human, horses, and other mammals through mosquitoes, it belongs to a faction of viruses called arthropod-borne viruses (arboviruses). Taxonomic classification indicates that WNV belongs to the family of Flaviviridae, the genus of Flavivirus, and its species name is West Nile Virus (Roehrig 3088). When infected mosquitoes bite humans, horses, and other mammals, they infect them with WNV and cause serious neurological disorders. Although 80% of the people infected with WNV remain asymptomatic, infected horses experience severe neurological disorders because they are highly susceptible to WNV (World Health Organization par. 1). Moreover, the young, the old, and people with compromised immune systems due to viruses such as human immunodeficiency virus or taking immune suppressive drugs are very susceptible to WNV. Common symptoms associated with WNV are fever, fatigue, malaise, anorexia, vomiting, muscle pain, vomiting, and nausea. These symptoms reflect neurological disorders such as encephalitis, meningitis, poliomyelitis, and acute flaccid paralysis, which are related to WNV. Fundamentally, WNV causes a myriad of neurological disorders that affect the functioning of the brain, the spinal cord, and the peripheral neurons.
Historical information shows that WNV is common in the Middle East, West Asia, North America, Europe, and Africa. However, scientists discovered its existence in 1937 when they isolated WNV from a Ugandan woman in the West Nile part of Uganda (World Health Organization par. 3). Further research led to the discovery of WNV in the birds such as Columbiformes and crows, which lived in Nile Delta region in 1953. During its discovery, scientists did not consider WNV as a pathogenic virus to birds, but a virulent strain emerged in Israel in 1997, which caused massive deaths of various species of birds (Roehrig 3088). The outbreak of WNV in the United States in 1999 revealed the virulence and severity of WNV among humans. Subsequent outbreaks of WNV in Russia, Greece, and Romania coincided with the migration and migratory routes of birds. Hence, the trend of the outbreak revealed that birds and mosquitoes are responsible for the spread of WNV across the world. Currently, WNV has spread across the world and has the potential of infecting humans, horses, and other mammals.
Classification, Viral Structure, and Viral Genome Organization
Given that WNV is a virus, taxonomic classification shows that it belongs to the phylum of viruses. Viruses are a group of organisms that are unable to replicate on their own for they need host cell machinery. Essentially, viruses enter into the host cell and use the protein synthesizing machinery in synthesizing their proteins and replicating their genetic material (Brinton 16). Moreover, WNV belongs to the class of RNA positive-strand viruses, which contain positive ssRNA, according to the Baltimore Classification. WNV belongs to the order called Flavivirales and falls in Flaviviridae family because it is an arbovirus. WNV is an arbovirus because its mode of transmission from birds to horses and other mammals occurs through the bites of mosquitoes. Specifically, Culex species of mosquitoes such as Culex tartsalis, Culex pipines, and Culex univittatus bite birds infected with WNV and transmit the virus to humans and horses where they cause diverse neurological disorders (Roehrig 3097). The genus name of WNV is Flavivirus while the species name is West Nile Virus.
As the structure of WNV, virions are spherical in shape and contain an envelope with receptors that they use in the attachment and entry into a host cell. The electron microscope shows that the diameter of a virion is approximately 50 nanometers with numerous receptors that surround the envelope. The virion has both exodomains and endodomains parts of the receptors, which span the membranes and form the envelope that encloses a virion. According to Brinton, WNV has a capsid that covers the genome and interacts with the protein membrane at the internal surface of envelope (14). The endodomains of the receptors interacts with capsid while the exodomains of the receptors interact with the host cells. The structure of WNV is similar to that of other viruses for it has the envelope, receptors, capsid, and the genetic material.
WNV has an organized genome in its nucleocapsid, which contains RNA. The size of the RNA is approximately 11kb, which is a single-stranded and positive sense (Brinton 15). WNV transcribes the RNA into mRNA and translates it into viral proteins within the host. The genome comprises both exons and introns, which are coding and non-coding regions of the genome sequence. The genome sequence of WNV comprises a 5’ intron of 96 bases and a variable 3’ intron of 337 to 649 bases with a conserved CUOH tail instead of poly-A tail that is common in other viruses (Brinton 15). The 5’and 3’ sequences of the genome sequence interact and form secondary structures, which are unique to flaviviruses because of their conserved structures. The deletion of 3’ and 5’ terminal sequences have deleterious effects on WNV because it prevents the formation of the conserved secondary structures.
Viral Replication Cycle
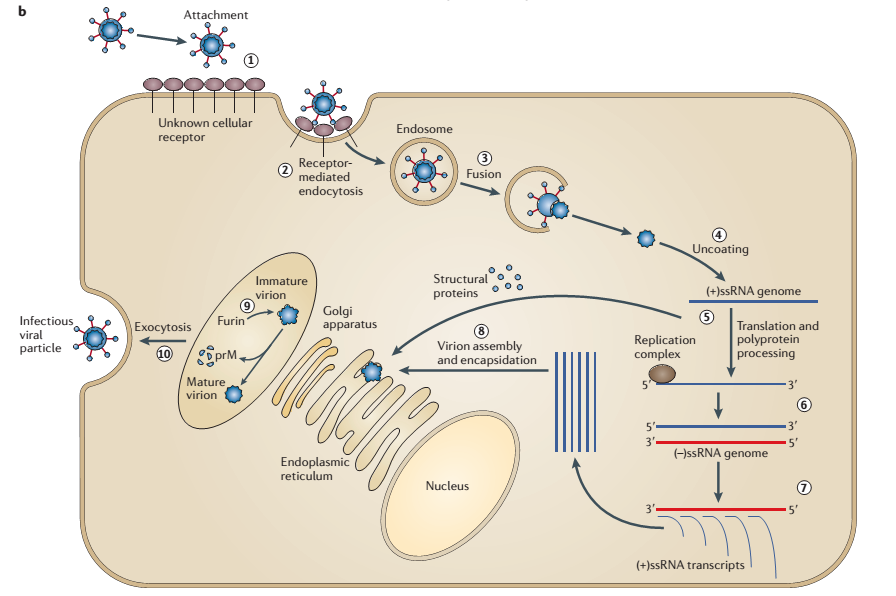
The first step in viral replication is the attachment of the viruses to the host cell using viral receptors. During attachment, viral receptors interact with cellular receptors of host cells such as mammalian, avian, and insect cells. According to Brinton, cells have co-receptors, which provide surfaces for viruses to attach and interact with the host cells in a cooperative manner (14). Although scientists have not established the exact mechanism of attachment, they believe that receptors of WNV interact with glycosaminoglycans on the surfaces of host cells and promote the entry of flaviviruses. The second step of viral replication is the entry of viruses into the host cells where they replicate. Colpitts, Conway, Montgomery, and Fikrig describe the entry process as receptor-mediated endocytosis (636). The entry mechanism is receptor-mediated because it involves the interaction of viral receptors and cellular receptors. Moreover, the entry involves endocytosis since the host cell invaginates and creates an endosomal vesicle (endosome) that carries a virion into the cell as shown in Figure 1. As the endosome matures, it becomes acidic and causes the envelope protein to fuse with the endosomal membrane and remove the coat of the virus. Subsequently, the virus releases its positive ssRNA into the cytoplasm of the host cell where replication occurs.
The third step of replication is a replication of positive ssRNA. When the endosome releases positive ssRNA, the replication complex undertakes the replication mechanism and produces numerous copies of negative ssRNA. Transcription of these negative ssRNA gives numerous transcripts of positive ssRNA, which WNV uses in the assembly and encapsulation of virions. The third step of viral replication is the translation of positive ssRNA to obtain structural proteins that the virus requires in the assembly and encapsulation of virions. Suthar, Diamond, and Gale explain that the translation machinery synthesizes a single polypeptide at the endoplasmic reticulum, and cellular proteases and viral serine protease cleaves the polypeptide into structural proteins (117). Fundamentally, the structural proteins that the translation process synthesizes are protein coat, capsid, receptors, and nucleocapsid proteins. The fourth step entails the assembly and encapsulation of genetic material to form virions. The encapsulated immature virions migrate through the endoplasmic reticulum and Golgi apparatus and enter into the exocytic vesicles (Colpitts et al. 636). The fifth and final step is the release of mature viruses through the process of exocytosis. The mature viruses are infectious and are ready to infect another cell by following the steps outlined above.
Transmission, Incidence, and Epidemiology
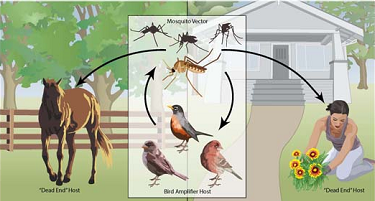
Although WNV affects some birds, other birds are resistant, and thus, they act as prime reservoir hosts. In the United States, the American robin is the prime reservoir host of WNV. When mosquitoes bite infected birds, they transmit WNV to other birds or mammals. In this case, mosquitoes act as vectors for they play a central role in the transmission of WNV from birds to humans, horses, and other mammals. Culex mosquitoes are the main vectors of the arbovirus because they can feed on numerous mammalian and avian species, hence, promoting the transmission of WNV. Although numerous species of mosquitoes can transmit WNV, the notable ones are Culex tarsalisis, Culex thriambus, Culex quinquefasciatus, Culex nigripalpus, Culex stigmatosoma, and Culex pipiens. According to Colpitts et al., Culex tarsalisis is a vector that is dominant in the western United States, and it transmits WNV to birds and mammals while Culex pipiens is common in the eastern United States and it transmits WNV to mammals and humans (636). The ability of mosquitoes to feed on both birds and mammals promotes the spread of WNV in the United States and across the globe.
Once a mosquito feeds on infected blood from the prime reservoir host, the viruses go into the midgut, replicate, and mature into viruses, which travel to the salivary gland via hemolymph. The infected mosquitoes can then bite humans, horses, and other mammals, which are dead ends because they cannot amplify the level of viruses in their bloodstreams (Centers for Disease Control and Prevention par. 2). However, when mosquitoes bite birds, they increase the transmission rate of WNV. The figure below illustrates mosquito-bird transmission, mosquito-to-horse transmission, and mosquito to human transmission of WNV.
The cases of WNV date back to 1937 when it was discovered in Uganda when it affected a woman. From Uganda, WNV spread to Israel, Egypt, Romania, the United States, Europe, Congo, Greece, and Canada where there were few cases of WNV reported in different regions. However, from 1999, the incidences of WNV increased considerably owing to the migration of birds. Roehrig reports that 37,000 cases of WNV have occurred in the United States since 1999, which has made WNV to be the primary root of encephalitis transmitted by mosquitoes (3100). However, the incidences of WNV have been fluctuating in the last 15 years since its major outbreak in the United States. In 2012, 5000 cases of WNV occurred in the United States, which is the highest level of incidences reported (Roehrig 3100). In Canada, Europe, Africa, and Asia, the incidences of WNV have been increasing with time owing to the ability of mosquitoes to feed on both mammals and birds. According to the Centers for Disease Control and Prevention, there were 2,060 cases of WNV that occurred in 2015 among 48 states in the United States (par. 1). The analysis of the cases reveals that 34% (700) constituted non-neuroinvasive diseases while the remaining 66% (1,360) constituted neuroinvasive diseases such as encephalitis and meningitis. Therefore, the incidences and epidemiological statistics indicate that WNV is recurring in the United States and across the world owing to the migration of the birds.
Clinical Manifestation and Mechanism of Disease
Most of the infections of WNV have no clinical manifestation in humans. According to World Health Organization, 80% of human infections are subclinical because most of the viral strains are mild and do not cause severe disease (par 1). However, in some rare instances, individuals infected present diverse clinical manifestations, which indicate that they have viral infections in their system. The clinical manifestations can reflect non-neuroinvasive or neuroinvasive effects of WNV. The non-neuroinvasive clinical manifestations are headache, fever, fatigue, excessive sweating, chills, nausea, diarrhea, and flu-like symptoms amongst others. The neuroinvasive clinical manifestations are encephalitis, meningitis, paralysis, poliomyelitis, hyporeflexia, and meningoencephalitis (Colpitts et al. 639). These neuroinvasive manifestations indicate that WNV causes neurological disorders by affecting the central nervous system.
Pathophysiology indicates that the mechanism of action of WNV is to invade the central nervous system, which is the brain and central nervous system, and cause neurological disorders. Lim, Koraka, Osterhaus, and Martina explain that WNV enters the brain and spinal cord where it multiplies and affects neurological functions leading to paralysis, encephalitis, and meningitis, which are some of the neuroinvasive clinical symptoms (813). Although it is extremely difficult for a pathogen to cross the blood-brain-barrier and enter into the brain and spinal cord, WNV employs some special mechanisms. These mechanisms are through passive transport across the blood-brain-barrier, olfactory bulb, infected immune cells of the central nervous system, and direct axonal retrograde transport. The severity of WNV is dependent on the state of immunity among individuals. According to Colpitts et al., individuals with compromised immunity, such as the aged and those taking immunosuppressive drugs, are susceptible to neuroinvasive effects of WNV. Moreover, cardiovascular diseases, immune diseases, hepatitis C, and renal disease predispose individuals to the neuroinvasive effects of WNV.
Control and Therapeutic Intervention
Given that mosquitoes are primary vectors that transmit WNV from the prime reservoir hosts, their control forms a first-line strategy. Methods of controlling mosquitoes to prevent them from biting humans and infecting them with WNV are the use of mosquito repellants on the skin, wearing protective clothing, spraying houses and the environment with insecticides, and draining water into the environment to prevent mosquitoes from breeding. Since there are no viable therapeutic interventions, early diagnosis is necessary so that infected individuals can receive supportive care in time to avert the occurrence of neuroinvasive disorders. Supportive care involves prevention of secondary infections, augmentation of immunity, the addition of intravenous fluids, and use of respiratory support (Colpitts et al. 642). However, the emergence of equine vaccines has proved to be effective in the prevention of WNV among horses. Therefore, the control of mosquitoes and the establishment of therapeutic interventions form the basis of preventing the occurrence of WNV among diverse populations across the world.
Current Inconsistencies and Debates
Among scientists, there are inconsistencies in the genus and species of mosquitoes that spread WNV. While most scientists recognize Culex species, few scientists recognize Aedes species as vectors of WNV. In this view, Colpitts et al. recommend the study of Aedes albopictus to enhance understanding of WNV so that it can open new ways of treating and controlling WNV in the population (642). The recognition of other species of mosquitoes would expand knowledge regarding replication and transmission of WNV from birds to mammals. Since the pathogenic mechanism of WNV is still unknown, scientists hold diverse views. Currently, there are four pathogenic mechanisms of WNV, which are infection of the epithelial cells through passive transport, infection of the olfactory bulb, infection of the immune cells of the central nervous system, and the infection of the peripheral neuron through direct axonal retrograde transport (Lim et al. 814). Hence, the existence of debates and theories regarding the pathogenic mechanism is still a challenge in the control, treatment, and prevention of WNV.
Conclusion
WNV is a virus that causes neurological disorders in humans and mammals. It belongs to the phylum of the virus, class of Flaviviridae, the genus of Flavivirus, and species of West Nile Virus. Since its discovery in 1937 in Uganda, WNV has spread to different parts of the world because birds such as the American robin are prime reservoir hosts who migrate across the globe. The common vector of WNV is Culex species of mosquitoes, which bite both mammals and birds, and thus, bringing about the transmission of WNV from one host to another. Epidemiological statistics show that WNV has high incidences in the United States, Canada, Israel, Congo, and Romania. WNV exhibit both neuroinvasive and non-neuroinvasive clinical manifestations when they infect humans. Although there are no current therapeutic interventions, control of mosquitoes and provision of supportive care help in the prevention and treatment of WNV infections. Therefore, to enhance the prevention and control of WNV, scientists study all potential vectors, elucidate the pathogenic mechanisms, and design drugs.
Works Cited
Brinton, Margo. “Replication cycle and molecular biology of the West Nile Virus.” Viruses 6.1 (2014): 13-53. Print.
Centers for Disease Control and Prevention. West Nile Virus.2015. Web
Colpitts, Tonya, Michael Conway, Ruth Montgomery, and Erol Fikrig. “West Nile Virus: Biology, Transmission, and Human Infection.” Clinical Microbiology Reviews, 25.4 (2012): 635-648. Print.
Lim, Stephanie, Penelope Koraka, Albert Osterhaus, and Byron Martina. “West Nile Virus: Immunity and pathogenesis.” Viruses 3.6 (2011): 811-828. Print.
Roehrig, John. “West Nile Virus in the United States: A historical perspective.” Viruses 5.12 (2013): 3088-3108. Print.
Suthar, Mehul, Michael Diamond, and Michael Gale. “West Nile virus infection and immunity.” Nature Reviews Microbiology 11.1 (2013): 115-128.
World Health Organization. West Nile Virus. 2011. Web.