Introduction
Medical laboratories are critical to human life. They are the place biological samples are tested and analyzed to facilitate screening, diagnosis, prognosis, treatment, and disease prevention. Typically, they are vital for patient care. They must be able to adequately fulfill patient and clinical personnel needs to be considered in conformance with quality guidelines.
Internationally set quality standards for laboratories outline both management and clinical practices that laboratories should adhere to. Routine laboratory practices must conform to the stipulated requirements with the guidelines. Personnel has a responsibility to ensure that these guidelines are adhered to. This paper will highlight quality issues arising from the laboratory which have either resulted in client complaints or do not conform to the provided clinical guidelines for laboratories the issues to be evaluated will include staff technical competence and delayed results.
Technical Competence
This dimension refers to the skills, capability, and actual performance of laboratory service providers, administrators, and support staff. The management has a responsibility to ensure that technically competent staffs are available to attend to patient needs and advice them appropriately (Grant & Leavenworth, 1988). For instance, a village laboratory worker must have the necessary skills and knowledge to be able to efficiently provide laboratory services.
Technical aspects cover the abilities of availed staff to execute practicable guidelines and standard reference terms in a dependable, accurate, reliable, and consistent manner. This is a dimension relevant to both clinical and non-clinical practices within a laboratory service setting. For instance, an inefficient records keeper would create long queues and long wait durations before patients are attended to. The same is true for an inefficient doctor. Technical services further refer to the availability of technical facilities which facilitate patient care (Grant & Leavenworth, 1988).
For example, an x-ray machine must be in a proper working condition other than just being available. Poorly maintained x-ray machines can result in result deviations which ultimately compromise patient safety. Machines should be handled by qualified staff. However, t is often practiced for various laboratories to let persons not certified handle tests. This is normally done when such a person has been doing some supportive role within the laboratory for some time and has consequently gained experience to perform minor tests.
Cause-and-Effect Diagram
The cause and effect diagram helps in the examination of various factors resulting in a given problem. Several steps are often involved in the development of cause and effect diagrams. These include;
- Problem traits identification
- Brainstorming reasons that could result in the problem.
- Grouping the identified reasons based on affinity technique.
- Developing a cause and effect diagram
Quality provision for patients under emergency care makes doctors from the emergency department reliant on constant laboratory tests. They entirely rely on laboratory tests to make decisions and hence such tests should be accurate and reliable in addition to being produced promptly. Often though, the turnaround period before feedback is presented to the doctors is delayed and results in delayed patient assistance (Grant & Leavenworth, 1988).
Cause and effect present a useful tool in the evaluation and improvement of the process to ensure reduced turnaround time. Turn around time to the process and reports generation for various emergency case tests is a critical function of healthcare provision. Delays cost lives and even lead to worsening of conditions that could have otherwise been controlled. This paper uses a cause and effect diagram to analyze the possible causes of such delays and hence propose ways of controlling them.
Analysis
In the analysis phase of this paper, a root cause analysis of delayed turnaround periods is performed using the cause and effect diagram (see figure below). The diagram helps in understanding the reasons which cause the long delays of specimens from the ED. These potential causes will form the basis for process rectification to ensure timely delivery of results.
Cause and effect diagram for turnover delays
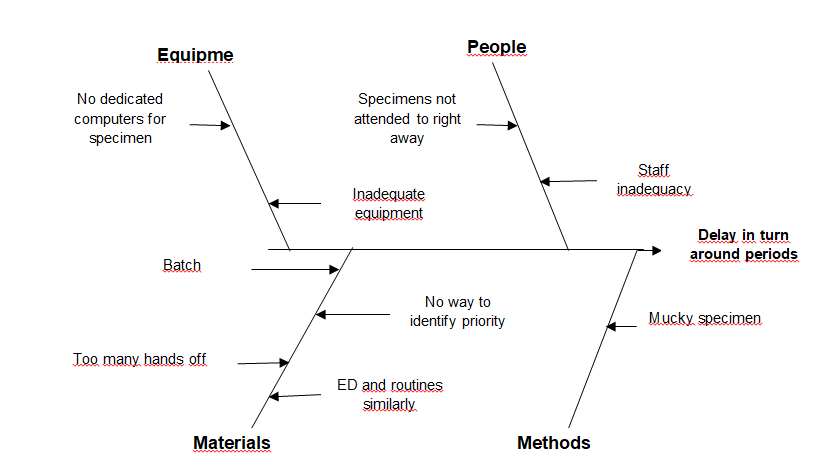
Potential solutions to the ideas proposed above were brainstormed and documented for purposes of rectifying the time delays. A prioritization matrix was used in identifying the implementation of the solution and which would yield the highest impact. The solutions were listed based on priority ratings and possible impact on solving the problem.
Proposed solutions:
- Process specimens from the emergency department in a single piece flow (Don’t batch)
- Process Specimens from emergency department separate from routine samples
- Use visual displays on centrifuges when running Specimens from the emergency department
- Set timers with audio alarms to spin time on centrifuges
- ‘Stat’ every specimen from the emergency department on the instruments
- Specimens from the emergency department must be processed by whoever is in the lab
- Quick response to specimens upon arrival to the lab.
- Reduce the number of handoffs in the workflow
- Dedicating a special computer and printer for purposes of processing Specimens from the emergency department
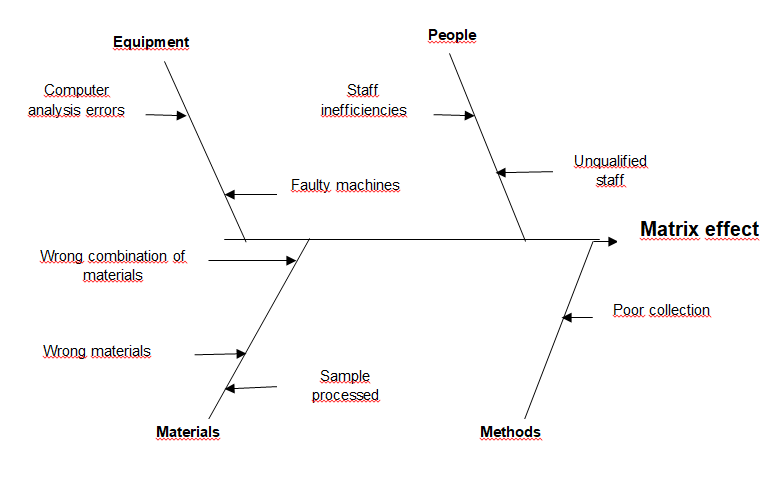
Matrix effect
Matrix effect is the sample property’s influence, independent of analyte presence, one measurement value. The sample matrix is made up of all material system parts, excluding the analyte itself. Matrix effects are either chemical or physical. Physical is a result of physical traits including viscosity and surface tension among others.
No-native analytes including enzymes not originating from humans can result in different signals from those traditionally expected. Control materials need to be similar to samples from the patient to keep matrix effects minimal. Often sample compositions as measured from laboratories come in varieties and hence rarely mimic the samples from patients under evaluation.
Matrix effects presence is an issue of concern to clinical practice over the years. Despite the existence of vast literature on fluid incompatibility, as well as procedures for measurement, identification, and quantification of matrix effects remains a major challenge and hence the need to ensure that it’s limited in occurrence. When in large quantity, matrix effect may bias the results and ultimately completely alter the results resulting in the wrong diagnosis.
Because of these, the effects are usually subject to quality assessment and protocols put in place to minimize their effects when they occur. Only then, can the laboratory guarantee that the results submitted for diagnosis are reflective of the actual patient’s condition. Only then can the laboratorian assess whether the observed effect(s) will have an impact on patient care (Tietz, 1987).
Determining the presence or absence of matrix effects allows users, manufacturers, and those responsible for evaluating EQA and PT data to distinguish between a true malfunction of the measurement procedure and incompatibility between the procedure and the material being tested. The real difference is that measurement procedure malfunctions affect patient care, while matrix effects limit how the procedure can be evaluated and monitored.
When matrix effects are present with procedure calibrators, calibrator values should be adjusted so that reported patient results are not affected. This has become standard practice among manufacturers.
If a difference in results between measurement procedures is observed with processed samples using these protocols, an interfering substance might be present. However, its source is not known in this early evaluation stage; it could be caused by a specific substance(s) or by the matrix—the milieu of the sample that differs from the specimens for which the procedure was designed. It could also be caused by differences between the analyte of interest and the actual measurand (the quantity that is intended to be measured).
We decided for this document to use the broadest interpretation; that is, this procedure is an effective way to identify whether an unexpected difference in results is observed in processed samples, and we direct the user to CLSI/NCCLS document EP7—Interference Testing in Clinical Chemistry to test the source of the bias and quantify its magnitude in terms of the analyte and interfering substances. The working group believes these protocols and the supporting information will be most useful to manufacturers and providers of external evaluation programs (Franzini & Ceriotti, 2004).
Our objective is to provide ways to identify the presence of matrix effects so that improvements in method specificity and fluid compatibility (controls and calibrators) can be made and to provide government regulators with a mechanism that can be used to distinguish between laboratories that are doing acceptable work from those that need improvement (based on the results of EQA/PT).
The working group anticipates that this guideline will be helpful when differences in results between measurement procedures are observed with control or proficiency test materials that might affect an understanding of method performance.
Trueness, traceability, and commutability are of current interest, collectively and independently, to help achieve consistent and accurate clinical measurements for patient benefit, regardless of where a measurement procedure is performed. The protocols in EP14 have been suggested as useful for identifying commutable materials.
Although we do see the potential for such use, we are cautious in recommending it without modification. Procedures to provide high assurance that material is intended as a “universal” calibrator must be assessed with greater rigor (more fresh patient specimens, more reagent, and calibrator lots, more runs) than these procedures provide.
This could be the objective of another guideline or as an addendum to future editions of EP14. Another method has been proposed recently to demonstrate the commutability of materials, with the use of inter-laboratory assessment schemes in which several measurement procedures are used routinely.
The general rationale used to develop each protocol was that clinical laboratory procedures are designed and developed to work optimally with patient specimens. Characteristics of manufactured control or calibrator materials that deviate significantly from the way patient specimens behave in specific procedures, with whatever response characteristics are used for measurement, can be called “matrix effects” because the source of the difference has not been identified (Franzini & Ceriotti, 2004).
Pragmatically, for this document, an observed difference of unknown source is called a “matrix effect,” while a difference due to an identifiable substance or physical characteristic is an “interference” (see Appendix A), and the user is referred to as CLSI/NCCLS document EP7—Interference Testing in Clinical Chemistry. Definitions are streamlined to account for known and unknown interferences.
The limitations of these protocols include (but are not limited to) the following:
- Subtle analytical differences that occur with consistency between different procedures for measuring a given analyte may not be easily detectable. These protocols may not be sufficiently powerful to detect or identify the presence of these differences. (Protocols described in Sections 6.3(6) or 6.4(2) could be helpful.)
- No attempt is made to determine the clinical or regulatory significance of the magnitude of difference or bias between measurement procedures. However, the magnitude of the bias or difference might be used to compare to independently derived clinical or regulatory (e.g., PT) limits.
- These protocols cannot determine which of the two procedures is more specific for measuring or for accurately detecting an analyte in a particular fluid.
- These protocols might not be used within all disciplines of clinical analysis.
Lastly, elimination of matrix effects requires either an improvement in the analytical specificity of procedures or the materials used for quality control, calibration, and/or external assessment. The clinical laboratory testing community should not lose sight of the fact that, in a perfect world, there would be no “matrix effect.” In such a world, every routine method would have sufficient analytical specificity to produce accurate results with any fluid or material. This lack of analytical specificity, however, is the reason this guideline is needed.
Importance of non-conformance testing in laboratory tests
Accurate, in-depth, and reliable investigations are critical to laboratory efficiency and hence functionality. Testing the integrity of laboratory equipment, processes and resources are of paramount importance during patient sample investigation. As a laboratory operative, it is important to ensure that laboratory evaluations are performed without compromising the quality and reliability of the same.
Results from the laboratory need to reflect the actual condition of the patient and capture all information necessary for a successful diagnosis of the patient’s condition. Failure to identify and make appropriate amendments to the root causes of deviations is a major concern to many regulatory bodies. Often such bodies expect that each laboratory has in place a proper and appropriate mechanism to evaluate its level of conformance to stipulated requirements. However, for purposes of surety, the regulatory authorities are often forced to perform non-conformance tests.
Laboratory tests are fundamental to correct the diagnosis and prognosis of patients. Nonconformance often compromises the results and put the life of the patients at risk. It is on this basis that medical professionals emphasize the need to constantly perform non-conformance investigations. Non-conformance investigations help in highlighting various critical aspects to the laboratory operatives as well as the management. Some important aspects to the management revealed by non-conformance tests include the under listed;
- Inadequate leadership/supervision
- Inadequate training
- Poor driver communication
- Poor management
- Poor management practices
- Inadequate work standards
- Other:
In addition to these, various process aspects which hamper efficient service delivery are identified include resources inadequacy, staff laxity, level of skilled staff available, and commitment by staff among others. Generally, non-conformance investigation offers a measurable approach to the evaluation of the efficiency of the laboratory facility. The management can identify the gaps in efficiency and appropriate take corrective actions to salvage the situations.
References
Franzini Carlo, Ceriotti F. (2004). Impact of reference materials on accuracy in clinical chemistry. Clin Biochem, 31, pp. 449-457.
Grant, E. L. & Leavenworth, R. S. (1988). “Quality Control”, Sixth Edition. London: McGraw-Hill Book Company.
Tietz, N.W. (1987). “Fundamentals of Clinical Chemistry.”New York: W.B. Saunder Company.