Objective
This experiment was carried out with the aim of separating various components of Phensuprin, which included sucrose, acetanilide, as well as through acid-base extraction process.
Characteristics of components used
Acetylsalicylic acid
Physical properties
Acetylsalicylic acid exists in the form of white crystals with a molecular weight of 180.16, derived from the component’s molecular formula C9H8O4. This acid has very high boiling and melting points of 140°C and 134-136°C, respectively. The figure below shows the structure of acetylsalicylic acid.
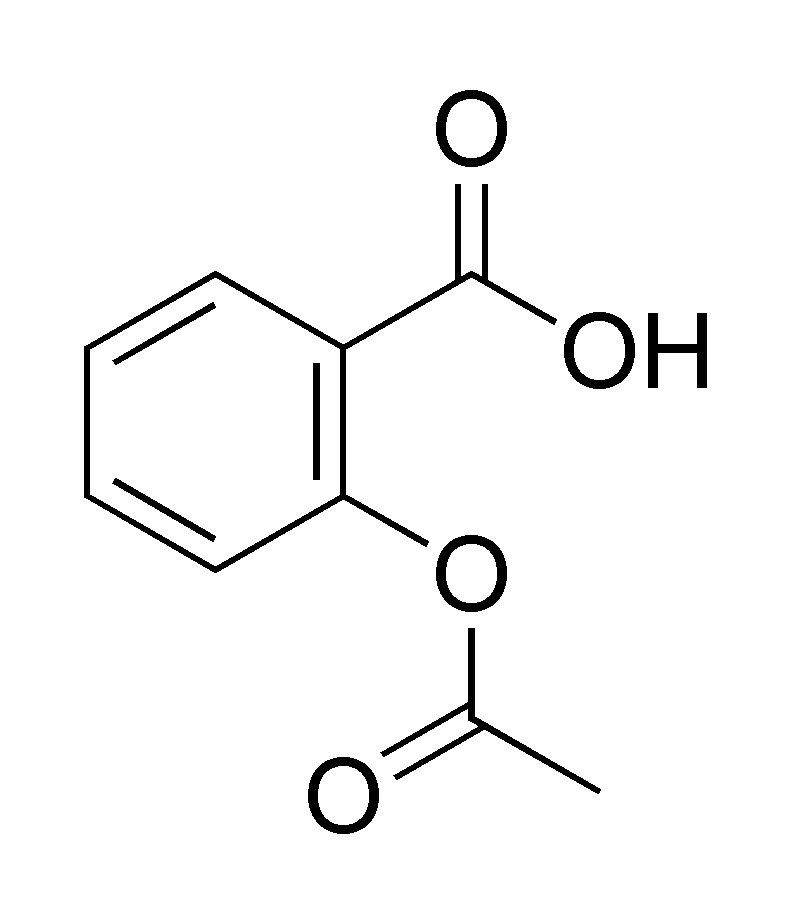
Acetylsalicylic acid is soluble in water with a solubility of 3.3g/L at a temperature of 20°C.
Hazards
Acetylsalicylic acid is highly flammable, irritating and poisonous.
Acetanilide
Physical properties
Acetanilide is a white or grey powder whose molecular weight is 135.16, and the molecular formula C8H9NO4. Below is a figure showing the molecular structure of acetanilide.
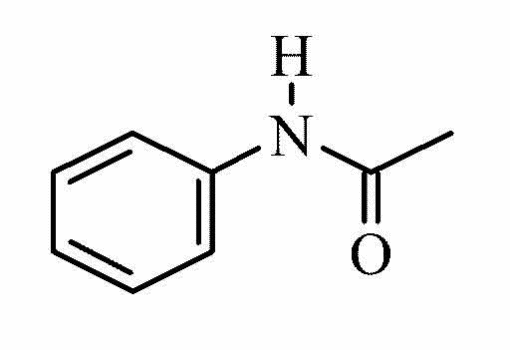
The melting point of acetanilide ranges between 113 and 115°C, while the boing point is 304°C. The solubility of acetanilide at a temperature of 20°C is 5g/L.
Hazards
Acetanilide has an irritating characteristic; it is toxic and hence, very harmful.
Sucrose
Physical properties
Sucrose exists in the form of powdered crystals with a molecular weight of 342.3 based on the molecular formula C12H22O11, as shown in the figure below.
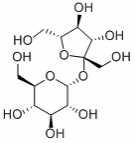
This component has a melting point that ranges between 185 and 187°C. However, the compound decomposes upon boiling. It is highly soluble with a solubility of 1970g/L at a temperature of 20°C.
Hazards
Sucrose has an irritating characteristic.
Dichloromethane
Physical properties
Dichloromethane is a liquid that is colorless in nature with a molecular weight of 84.93 based on the molecular formula of CH2Cl2, as shown in the figure below.
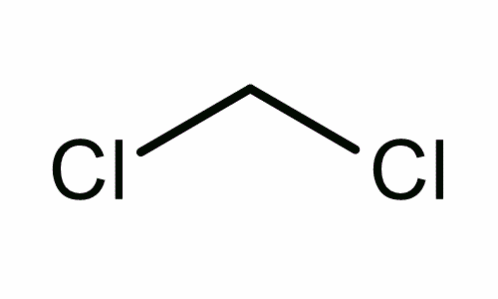
Dichloromethane has a very low melting point of -97°C and a boiling point that ranges between 39.8°C and 40°C. Its solubility rate is 20g/L at a temperature of 20°C.
Hazards
Dichloromethane is corrosive, carcinogenic, highly flammable, toxic and harmful.
Sodium bicarbonate
Physical properties
Sodium bicarbonate exists in the form of power or even crystals that are white in color. It has a molecular weight of 84.01 and a molecular formula of CHNa02, as shown in the figure below.
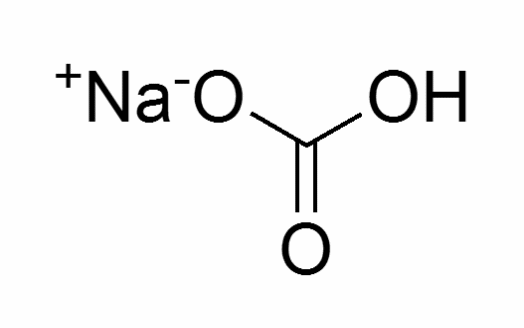
This compound has a very high melting point of 300°C and a boiling point of 851°C. Its solubility rate is 90g/L at a temperature of 20°C.
Hazards
Bicarbonate is harmful as well as irritant.
Sodium sulfate
Physical properties
Sodium sulfate exists in the form of power or crystals that are white in color. It has a molecular weight of 142.02 based on the molecular formula of Na204S, as shown in the figure below.
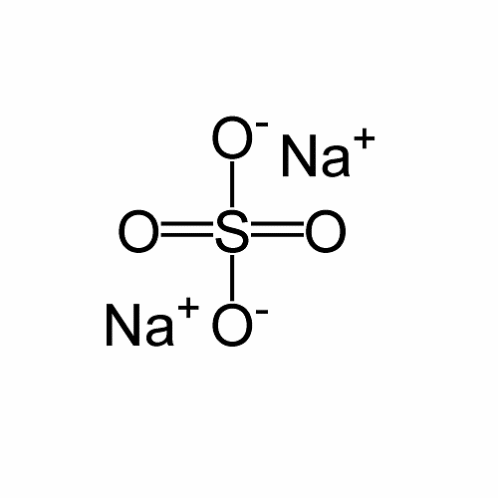
The melting and boiling points of sodium sulfate are very high; 884°C and 1700°C respectively. Resultantly, the solubility rate is low-18.5mg/L at a temperature of 20°C.
Hazards
Sodium sulfate has irritating characteristics.
Hydrochloric acid, (“Chemical Book: Product Chemical Properties” par. 2)
Physical properties
Hydrochloric acid is a liquid that has fuming characteristics, with a molecular weight of 36.46 based on the molecular formula of HCl, as shown in the figure below.
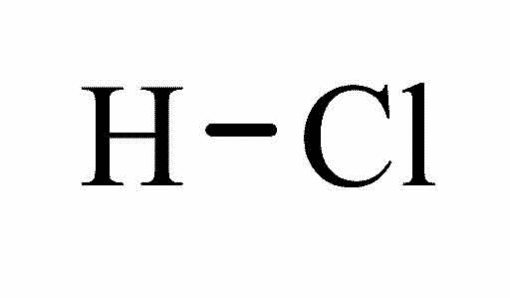
Tanabe observed that the melting and boiling points of hydrochloric acid are very low, -35°C and 57°C respectively (19). As a result, the compound is miscible.
Hazards
Hydrochloric acid is corrosive, toxic, irritant, harmful and extremely flammable.
Experiment
Reagent and materials
Procedure
A weighing balance was used to weigh 2g of Phensuprin sample and put in an Erlenmeyer flack (125ml). To the flask containing the sample of Phensuprin, exactly 50ml of dichloromethane were added. Using a rod, the mixture was stirred vigorously until all the particles of Phensuprin dissolved in dichloromethane.
A dry filter paper was measured and its weight recorded. This filter paper was to be used in measuring the weight of the solids used during the experiment. Next, the already weighed filter paper was folded accordingly and for use in filtering the mixture of Phensuprin and dichloromethane via the gravity filtration process that has the capacity of separating solids in a mixture. 5 ml of dichloromethane was used to wash the collected solids in the filter paper before drying the contents (sucrose) over a beaker at room temperatures for one week. After the contents were completely dry, the mass of the sucrose was determined by weighing both the filter paper and its contents. The recorded weight of sucrose was used to determine the percentage composition of sucrose in the 2g sample of Phensuprin.
The filtrate obtained from the filtration process contained significant percentages of acetanilide and acetylsalicylic acid. As such, the two compounds were separated through the extraction process. A separatory funnel was used, whereby the dichloromethane solution was put and extracted two times through the use of double portions of a solution of sodium carbonate (5%). The first part of the extraction process involved 25 ml of sodium carbonate solution, which was added in the separatory funnel containing the solution of dichloromethane, and the funnel stoppered, and slightly shaken for about 2 minutes. Intermittently, it was necessary to expel any pressure that had built up in the funnel.
Using a clamp, the funnel was fixed in such a position that the separation layers could occur. A 125ml Erlenmeyer flask was used to collect the dichloromethane drained from the lower layer in the separatory funnel. The top layer contained aqueous compounds and was thus, emptied into a 400ml beaker. The contents of the Erlenmeyer flask (dichloromethane solution) were put back into the separatory funnel for the second phase of extraction in the presence of 25ml of sodium carbonate. After separation, the aqueous phases were put together for the purpose of further extraction, while the dichloromethane phase was kept.
An ice bath was used for the purpose of cooling the aqueous phase from the previous extraction after which, 10ml of concentrated hydrochloric acid was slowly added through the use of a transfer pipette and stirred. Foaming occurred which necessitated vigorous swirling of the contents in the beaker as the acid was continuously added. A pH paper was used to determine the pH value of the resultant solution after the hydrochloric acid was completely added. This was done through the use of a stirring rod whereby a drop of the resultant solution was transferred periodically up to when the pH value of the solution was less than 2. The ice bath was used to cool the aqueous phase further in order to form acetylsalicylic acid in solid form, which was collected through vacuum filtration. For several minutes, the vacuum pump was run subsequently to the filtration process for the purpose of ensuring that the collected solid was completely dried. An uncapped sample vial was used to dry the collected solid at room temperatures for one week, after which it was dried and weighed to determine the percentage composition.
An anhydrous sodium sulfate was used to dry the dichloromethane solution for the purpose of extracting acetanilide. A scoop full of anhydrous sodium sulfate was added to the solution of dichloromethane and swirled to mix. Gradual additions of portions of sodium sulfate was carried out with an allowance for clumping until there were signs of excess sodium sulfate, which could be noted from free solids. A pre-weighed filter paper was used to filter the mixture in a 100ml round-bottom flask after subsequent swirling for several minutes. A rotary evaporator was used to evaporate the methylene chloride (the solvent) to leave acetanilide solid component. Unstoppered round-bottomed flask was used to collect the filter solid for use in subsequent lab periods. The collected solid was weighed for a chance to determine its percentage composition in terms of mass.
Lastly, melting points of the three compounds, eth acetanilide, acetylsalicylic acid and sucrose were used in the determination of their purity. In addition, a comparison of the literature melting points and the obtained melting points was done for a chance to note any discrepancies.
Results of the extraction process
The mass of the Phensuprin was 2.0g
Mass of the filter paper used to separate the sucrose was 0.97g
Combined mass of the filter paper and sucrose was 1.51g
Mass of sucrose obtained = combined mass – mass of filter paper
= 1.51g-0.97g
=0.54g
Mass of acetanilide obtained was 0.39g
Mass of the round bottom flask was 51.66g
Mass of acetylsalicylic obtained was 0.66g
Total mass: 0.54 + 0.39 + 0.66 = 1.59g
Total percent recovery; 1.59/2.0*100= 79.5%

Results’ Synopsis
The extraction process was successful and led to the extraction of 1.59g of recovered compound in comparison to the 2g of Phensuprin sample that was initially used, accounting to 79.5% recovery. Only an insignificant percentage of the Phensuprin sample initially used (20.5%) was not recovered during the extraction process. The lack of total percentage extraction was attributable to several errors during the process of extraction. Some of the possible errors during the extraction process include addition of more or less quantities of the reagents other than the required quantities. Secondly, errors in reading the weights could have had a significant effect on the final readings and hence, the recovery amount. Additionally, possible changes in temperature could have influenced the readings of the extraction process. The mass of the acetanilide, acetylsalicylic acid and sucrose obtained from the extraction process was 0.39g, 0.66g and 0.54g respectively. Out of these quantities, it was found out that the percentage composition of acetanilide was 24.53%, while that of sucrose and acetylsalicylic acid was 33.96% and 41.51% respectively of the total mass of Phensuprin sample that was recovered from the entire process.
The investigation of the melting points of the compounds showed that sucrose was pure due to the fact that the melting point according to empirical evidence and the obtained one were the same (185-187°C). On the other hand, it was noted that the melting point of acetylsalicylic acid was quite lower (127.4°C) compared to the literature value of 134-136°C, while the acetanilide compound’s melting point was 105.7°C, which was also lower than the literature value of 113-115°C. As such, it was observed that acetanilide and acetylsalicylic acid were not completely pure as they contained some percentage of impurities.
Discussion
The primary objective of carrying out this extraction experiment was to investigate the possibility of separating components successfully. As such, acid/base extraction process was used on Phensuprin sample. This process focused on the separation of compounds based on their differences in solubility whereby solid phases were extracted form liquid phases. The sample used, Phensuprin can be defined as an analgesic compound that comprises of percentages of acetanilide, acetylsalicylic acid and sucrose. Sucrose has a high solubility rate in inorganic solvents, while the acetylsalicylic acid is highly soluble in organic solvents. Such difference in solubility ability in organic and inorganic solvents was very instrumental in the separation of the components of Phensuprin. For this reason, Phensuprin sample was mixed with dichloromethane, an inorganic solvent, for the purpose of dissolving acetylsalicylic acid and acetanilide while hoping to extract sucrose as an insoluble solid. This was possible because sucrose is insoluble in dichloromethane and thus, it was possible to separate the solid through gravity filtration.
A study by Zubrick indicated that filtration is a physical process that is used in the separation of solids from the liquids through the use of a sieve or any other membrane that has the capacity of allowing the passage of liquids while at the same time restricting the passage of solids. In the case of this experiment, a filter paper was a suitable material for use to separate sucrose from the solution of dichloromethane. This was attributable to the fact that filter papers are porous and hence, can allow solution to pass while at the same time retailing any solid particles.
Dichloromethane was used to wash the collected sucrose for the purpose of removing traces of acetylsalicylic acid and acetanilide, which are potential impurities of sucrose. According to Povar and Spinu, washing is an important step in the filtration process as it is important in the removal of unwanted soluble materials from a residue collected. Failure to wash the collected residue would have led to impure sucrose due to the possible retention of elements of acetanilide and acetylsalicylic acid. Acid/base reaction was used in the manipulation of the solubility of acetylsalicylic acid as well as that of acetanilide. In spite of the fact that these two components of Phesuprin are soluble in dichloromethane, the addition of sodium carbonate changes their solubility. Eyal noted that sodium carbonate has the ability of deprotonating acetylsalicylic acid to sodium acetylsalicylate salt.
On the other hand, sodium carbonate is not able to react with acetanilide due to the fact that it is a weak base. For this reason, adding sodium carbonate makes the acetylsalicylic acid insoluble in dichloromethane. As such, acetanilide remained in dichloromethane solution. When sodium carbonate solution was added, conversion of the acetylsalicylic acid to sodium acetylsalicylate occurred, which is soluble salt.
Two layers of dichloromethane solution and sodium acetylsalicylate solution are formed because sodium acetylsalicylate solution is inorganic while the dichloromethane is organic solutions, which enhances their separation. In addition, Soldatov and Zelenkovskii noted that dichloromethane solution is much denser when compared to sodium acetylsalicylate solution, which ensured that the former formed at the upper side of the funnel while the latter formed at the lower part of the funnel.
Therefore, the lower layer contained acetanilide, which made it easy for the acetanilide to be drained easily. Sodium carbonate was added for the purpose of extracting the remaining acetylsalicylic acid. Hydrochloric acid was used in the extraction of acetylsalicylic acid since it acidified sodium acetylsalicylate through a reaction that formed acetylsalicylic acid, insoluble. On the other hand, keeping the pH of less than 2 was strategic for re-protonation of sodium acetylsalicylate, and thus, it was possible to precipitate out of the solution upon cooling in an ice cold bath (Cox, Siwick and Bakker 236-244). Anhydrous sodium sulfate was used in the extraction of acetanilide from the dichloromethane solution. The choice of the anhydrous sodium sulfate was based on the face that it is a drying agent. Zubrick noted that anhydrous salts are drying agents since they have the ability of absorbing water from diverse substances. In addition, the use of the rotary evaporator allowed the removal of dichloromethane from acetanilide solution since it is an inorganic solvent.
Based on the results of the experiment it can be considered that the separation of compounds was progressive due to difference in the compounds’ solubility. For example, sucrose was the first compound, which was separated using gravity filtration followed by the acetylsalicylic acid, which was separated through the vacuum filtration process. Povar and Spinu attributed this to the fact that acetylsalicylic acid because it is not only fast, but also acts as a drying agent. In comparison to the gravity filtration, it was noted that vacuum filtration is quite fast since it makes the use of vacuum pressure in filtering as well as to dry residues.
According to the results of the extraction process, it was evident that acetanilide comprised of half of the recovered Phensuprin, while Acetylsalicylic acid was the second and lastly, the sucrose component. The melting point of sucrose was the same as that of literature indicating that it was pure. However, the melting point of acetylsalicylic acid was 127.4°C and that of acetanilide was 105.7°C. Since these melting points were lower than the literature values, they indicate that acetylsalicylic acid and acetanilide recovered had impurities, which lowered melting points.
The difference in the percentage composition of the recovered compounds was attributed to experimental results such as in the process of washing sucrose residues, the separation of the organic and aqueous layers, as well as in the determination of the change in pH through the use of sodium carbonate and hydrochloric acid. In addition, temperature changes might have affected the extraction process. For example, lack of efficiency in washing the sucrose could have left some acetylsalicylic acid and acetanilide in sucrose hence, increasing the composition of impurities. In addition, the availability of interface on the aqueous and organic layers led to the introduction of impurities into either layer thereby, affecting the purity of the extracted compounds. The process of manipulating the pH value was aimed at the determination of the solubility of acetylsalicylic acid. As such, there was a high possibility of reduction in the acid’s solubility in a case of inappropriate manipulation.
Questions
The original organic solution was extracted two times with aqueous sodium bicarbonate. After the extraction, what compound(s) were in the organic layer? What compound(s) were in the aqueous layer?
The aqueous layer contained acetylsalicylic acid while the organic layer contained acetanilide.
Assume that both acetylsalicylic acid and acetanilide are soluble in diethyl ether, and that diethyl ether was used in place of the methylene chloride. Would the ether layer be the bottom layer in the separatory funnel or the top layer? Explain your answer.
Diethyl ether is less dense than water and methylene chloride and thus, the top layer would be diethyl ether.
Assume that both acetylsalicylic acid and acetanilide are soluble in methanol, and that methanol was used in place of the methylene chloride. What problem(s) might occur during the extractions? (Careful, this is a trick question.)
Methanol has the capacity of mixing with water to form a homogenous mixture of acetylsalicylic acid and acetanilide since it is a polar solvent. As such, it would be impossible to separate the two compounds since no layers would be formed as both acetylsalicylic acid and acetanilide will be in a same solution.
Historically, the solid residue that is left after the methylene chloride solution is evaporated has a lower melting point than its literature value, due to impurities. Explain what impurities are present and why.
Some of the likely impurities components of anhydrous sodium sulfate, acetylsalicylic acid, as well as sodium carbonate. This can be attributed to the fact that the process of extracting these components is not perfect and can be affected by other factors including change of temperature and human errors thereby leading to traces of the reagents used remaining in the previous mixture.
References
Cox, Jocelyn, Bradley Siwick, and Huib Bakker. “Influence Of Ions On Aqueous Acid- Base Reactions”. ChemPhysChem 10.1 (2009): 236-244. Print.
Eyal, Amos. “Cheminform Abstract: Acid Extraction By Acid-Base-Coupled Extractants”.ChemInform 28.52 (2010): 20-56. Print.
Povar, Igor, and Oxana Spinu. “Acid-Base Buffer Properties Of Heterogeneous Multicomponent Extraction Systems”. Solvent Extraction and Ion Exchange 33.2 (2014): 196-209. Print.
Soldatov, Victor, and Martin Zelenkovskii. “Interionic Interactions In Carboxylic Acid Cation Exchangers On The Base Of Polyacrylic Acid. Ab Initio Calculations”. Solvent Extraction and Ion Exchange 29.3 (2011): 458-487. Print.
Tanabe, Kozo. “Solid acids and bases: their catalytic properties”. Elsevier, 3.1 (2012): 19-29. Print.
Zubrick, James. The organic chem. Lab survival manual: A student’s guide to techniques. New York: John Wiley & Sons, 2012. Print.