Introduction
In standard chemical analysis methods, traditionally used in research laboratories for quantitative and qualitative measurements, techniques based on measuring electrical forces in chemical solutions are of great importance. Among all electrochemical methods of analysis, amperometry plays a prominent role, and for several decades has enabled laboratory engineers to conduct research not only in the areas of analytical chemistry but also in interdisciplinary ones: on integration with biology, physics, and medicine.
At the same time, it should be recognized that although the classical principle of operation of amperometric plants remains unchanged, modification of electrical laboratory instruments to give them additional properties is becoming increasingly common practice in the international scientific community.
Electrochemical Methods of Analysis
First of all, it should be recalled that the use of electrochemical methods in quantitative analysis is based on the use of dependencies of measured parameters of electrochemical processes on the content or concentration of the determined substance in the analyzed solution participating in this electrochemical process — whether it is the difference of electric potentials, current, or a quantitative measure of electricity. Thus, the basis of this area of chemistry is the direct quantitative measurement of electrical quantities in order to connect the obtained data with the chemical bases of electricity. Using the terminology of classical chemistry, electrochemical processes are traditionally understood as any molecular or atomic processes that are accompanied by simultaneous chemical reactions and changes in the system’s electrical properties, which in such cases is usually called an electrochemical system. In analytical practice, an electrochemical system usually contains an electrochemical cell, including a vessel with an electrically conductive analyzed solution into which metal or inert electrodes are immersed.
The Essence of the Amperometric Measurement
Consequently, amperometry is a special case of electrochemical technology. This method is based on measuring the ultimate diffusion current passing through the solution at a fixed voltage between an indicating and reference electrode. The nature of the formation of this current is determined by the chemical interaction between substances. Thus, electric current arises as a result of electrochemical oxidation or reduction of the electrically active substance at the application of potential pulse on the indicator electrode (Amine et al., 2018). In this case, the measuring medium can be a fixed stirring solution, flow, or rotating disk electrode. In the same way, as it is peculiar to volt-amperometry, in the given technique, the accent is put on the work of the small electrochemical cells serving as gauges on an output of columns of liquid chromatography.
More specifically, in the process of amperometric analysis, after adding individual portions of the reagent, the current strength at a voltage corresponding to the value of the diffusion current limit is measured. Thus, if an external voltage is applied to electrochemical cell electrodes, which exceeds the value of their electrode potential, the dynamic balance between direct and reverse chemical reactions near the electrodes is broken, and through the element begins to flow electric current. Moreover, it is necessary to say that at first, this current quickly decreases with time: this effect is caused by the fact that ions which are near the electrode immediately enter into a chemical reaction, as a result of which their concentration here quickly decreases and becomes much lower than in the main volume of electrolyte. That is why due to the decrease of ions concentration near the electrode reaction speed and electric current caused by it or, in other words, the flow of electric charges decreases.
The gradient of ions concentration in the electrolyte arising due to impoverishment near the cathode region stimulates diffusion of ions from volume to electrolyte. This effect is additionally excited by the added weak electric field, which is formed in the electrolyte. With time in the chemical medium, a new dynamic equilibrium is established where the number of ions entering chemical reactions near the electrodes in a unit of time is compared with the number of ions that approach the electrodes from the electrolyte volume through diffusion (Amine et al., 2018). Hence electric current ceases to change: numerically, it becomes proportional to the concentration of ions in the electrolyte. Therefore, measuring it after appropriate calibration of the sensor, it is possible to determine the concentration of ions in the solution under study quite accurately. Amperometric research is characterized by higher accuracy and sensitivity than the direct voltammetry method. Moreover, the possibilities of this method are significantly expanded, as the target processes can be sedimentation reactions, complexation, and oxidation and reduction mechanisms.
Motivation
The choice of this electrochemical method of analysis is primarily due to the author’s internal desire to study the essence of the amperometric measurement more deeply and to assess the limits of its practical applicability. Over the past few years, more relevant articles have appeared in the world’s biological and medical journals on the possibilities of using amperometry as an application for qualitative or quantitative evaluation of heavy metals concentration in tap water samples, research in brain neurophysiology, and papers on engineering modification of existing methods for recording analytical signals. Based on the data, amperometry appears to be a very promising method of chemical analysis, which, however, has rarely been properly influenced by specialists before. Summarizing the above, it should be noted that the internal desire to go deeper into the essence of the problem, as well as the desire to discuss promising areas of amperometry, have become decisive factors in choosing this area of electrochemical analysis for scientific research work based on the selection of literature.
Three papers demonstrating the versatility of applying the amperometric measurement in different areas of knowledge were chosen as the main articles for the research. Thus, the work of Dalkıran shows that amperometry can be used as an application for environmental monitoring of sewage samples for the assessment of heavy metals presence (2020). In addition, Wua et al. showed that in order to achieve the objectives of amperometry, it is possible to use multiplexed systems that allow data to be recorded in the flow of matter.
Finally, Antunović et al. showed that the amperometric measurement could also be applied to pharmacological studies of concentrations of active medicinal substances, including those found in beverages (2018). All together, these studies report but do not limit the range of applicability of classical amperometric devices in modern science. In addition to these authors, the work has made several references to other studies that allow a deeper understanding of the potential of amperometry.
Experimental Approach
Amperometry in Environmental Monitoring
The presence of heavy metal ions in water resources is a serious environmental problem. The presence of mercury, cadmium, lead, copper, and other metals in drinking water leads to serious pathophysiological changes in humans, animals, and plants, so it is necessary to develop methods for their qualitative and quantitative determination. The Dalkıran article showed that advanced and already applicable methods for estimating heavy metals in natural environments are very expensive and difficult to implement, while the use of modified enzyme inhibition systems facilitates the research process (2020). In particular, Dalkıran has demonstrated the ability to develop a simple and fast biosensor that inhibits the horseradish peroxidase enzyme activity to determine the presence of heavy metal ions — namely divalent cadmium, lead, and nickel — in tap water samples. The obtained materials had high selectivity, sensitivity, and stability, making such biosensors a promising solution for environmental monitoring tasks.
For this study, the range of organic and inorganic substances used both as main target components and as additives was used. The basis of the experiment was the horseradish peroxidase enzyme, which represents their biologically active substance with relatively small size, stability, and low cost in comparison with alternatives, including alkaline phosphatase. Among others, horseradish peroxidase is widely used in molecular biology research to amplify the weak signal to the level required for detection. However, it is known that the presence of heavy metals significantly reduces the activity of this enzyme: this property has been used for measurements. Special attention should be paid to the necessity of using nanoparticles of indium and tin oxide combined with nanoscopic ruthenium in this technique. Dalkıran argued that nanoparticles have a stimulating effect on electron transfer between electrodes and are also characterized by high electrical conductivity and biocompatibility. In other words, this additive was driven by the desire for a more sensitive biosensor.
The experimental basis for this study was based on the use of an electrochemical system consisting of three electrodes: Ag|AgCl for reference one, platinum, and glassy carbon electrodes. Thus, nanoparticles (5.9 mg) of indium tin oxide, ITO, as well as (3.4 mg) ruthenium, the effective size of which did not exceed the 50 nm mark, were sequentially submerged in the glassy carbon electrode matrix. Before that, the nanomaterials were pre-dispersed in 5 mg/mL of chitosan solution and dried at room temperature. Horseradish peroxidase enzyme was added to the resulting mixture on the electrode surface, as well as Nafion, which is a branched fluorocarbon chain ending in a sulfonic group. Ultrasonic cavitation effect was used for crushing the formed agglomerates and achieving more homogeneous mixing. The resulting biosensor, shown in Figure 1, was air-dried for some time, and after it was used to qualitatively assess the presence of heavy metals in tap water samples.
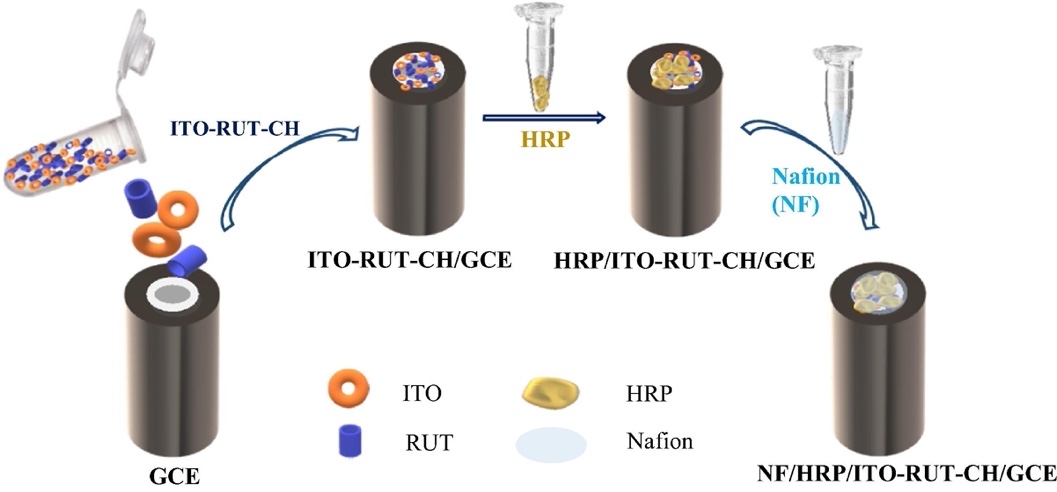
The specific mechanism of enzyme inhibition was to immerse the electrochemical system in 0.05 M saline solution with a phosphate buffer. Registration of the primary electrical signal (current) to create the baseline was replaced by the addition of a known amount of hydrogen peroxide in the medium, after which the current was also recorded. Enzyme inhibitors, which are heavy metals, were added to the solution and recorded the decrease in the numerical value of current. It was found that the increase of inhibitor concentration led to a decrease in the resulting current of the electrochemical system.
Multiplexed Amperometry
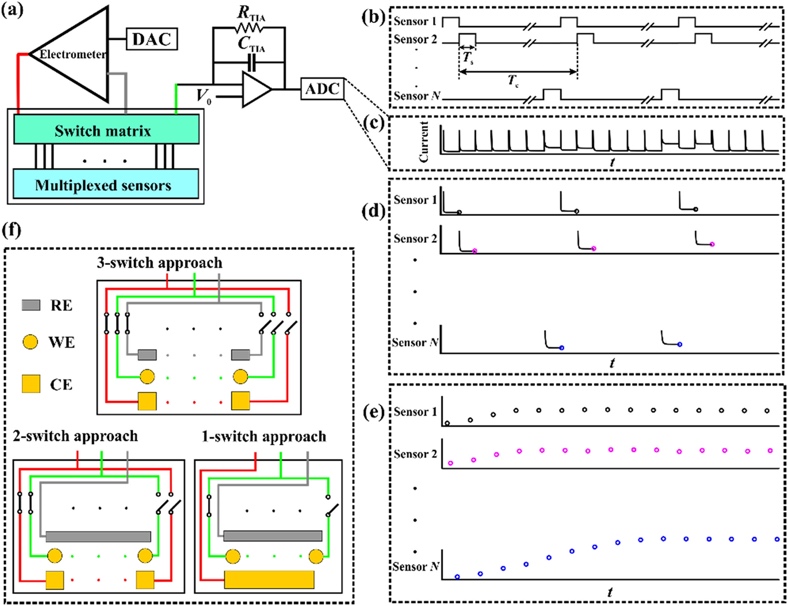
The central idea of this study of American scientists was the acceptability of scaling of received analytical signals emitted by electronic processes inside a chemical reaction. The experimental basis was the use of single-chip potentiostat, which provides measurements in electronic ELISA test. At the same time, the authors have shown that the allowable current is significantly lower than the defined level, so the circuit diagram was slightly modified. Two types of multiplexed biosensors were manufactured for this study, the difference between which was the composite structure. The first sensor was represented by five electrochemical systems, each with three electrodes, rounded, squared, and reference, and the second one by seven. The method of creating the electrodes was as follows: the working electrodes consisting of pyrex were sprayed with nanoscopic titanium and gold, while the reference electrode was represented by the classic Ag|AgCl variant or gold electrode.
Measurement of the signal set from potassium hexacyanoferrate was carried out in a flow mode by a sequence of sensors. At the same time, sixteen-time (16) multiplexed amperometry was provided by connection of a third-party device. A separate area of study was the measurement of signals from synthesized beads dissolved in a salt phosphate buffer, with each bead preliminarily mixed with samples of BSA or calf serum with the addition of human IL-6. The flow solution consisting of modified beads was measured with a sequence of sensors on both the gold and silver chloride reference electrode.
Pharmacological Research
From the above sections, it became clear that amperometry can be applied in different areas of knowledge, both in constant potential mode and in pulsed or multipulse modes. The latter variant has become the main focus of this study, aimed at evaluating the concentration of a medicinal substance in beverages. Auxiliary components of this experiment were buffer solutions, prepared both from mineral salts and from organic acids.
Cyclic volt-amperometry became an instrumental method for signal evaluation, and the electrochemical system consisted of three electrodes: a working one (glassy carbon), a silver chloride reference electrode, and a platinum wire. As in the Dalkıran experiment, the glassy carbon electrode was cleaned by rubbing a diverse aluminum paste. Three pulses were successively applied to the system and the resulting signal was calculated as the difference between the mean values of the first and second measurement phase.
Results
Amperometry in Environmental Monitoring
Although the specificity of the Dalkıran work lies in the multi-complex study of the synthesized biosensor — whether it is the measurement of surface morphology by emission microscopy and X-ray imaging, the evaluation of the effect of hydrogen peroxide concentration, or the choice of reference electrode — the basis of the measurement was the study of the principle of such an electrode for the detection of heavy metals. Thus, measurements on horseradish peroxidase inhibition showed that the modified electrode had an oxidation peak at -0.15 V and a reduction peak at -0.23 V. In general, these data testify to oxidation-reduction processes in ruthenium nanoparticles, actively used as an electron acceptor. It was also shown that the addition of hydrogen peroxide in the buffer solution had a positive effect on the electrocatalytic activity of the electrode.
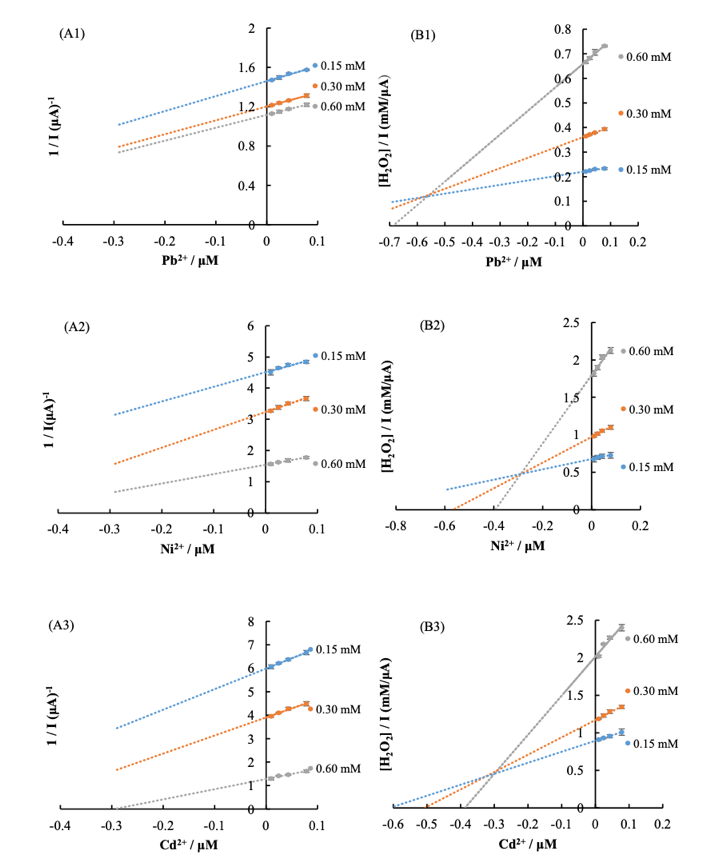
The expected question for Dalkıran was how the surface composition of the modified electrode influenced its activity. By changing the variable concentrations of nanoparticles, the author found that the optimal ITO concentrations were 5.9 mg/mL, and for ruthenium, 3.4 mg/mL. At the same time, increasing these concentrations, Dalkıran observed the intensification of the current response, but continuing the increase, this value sharply decreased. The core of the experiment was estimating the permissibility of the use of such electrodes for the detection of heavy metal ions. The researcher showed that adding even small amounts of lead, namely 10-4 M of a metal solution, led to the expected decrease of catalytic current. At the same time, it is important to note that the simultaneous detection of cadmium, nickel, and lead was not acceptable, because as shown in Figure 2, horseradish peroxidase inhibition is uncompetitive.
However, in the general case, the use of such a modified biosensor was successful because in the experiment to study the presence of heavy metals in tap water, the results were obtained. In particular, due to the electrode, detection of divalent cadmium, nickel, and lead was possible even below the WHO threshold. The data were statistically evaluated, and after that, Dalkıran concluded that it was possible to use the electrode as a new water pollution sensor.
Multiplexed Amperometry
The use of serial multiplexed device channels was the optimal solution for almost simultaneous reading of sample signals. The authors have shown that the analysis time in classical amperometry is much higher than in modern electronic devices, which means that the devices can be used for a detailed assessment of flow solutions (Figure 3). Thus, the measurement results from all sensors are recorded by the same analog-to-digital converter, which saves time and resources of the experiment significantly. The researchers also considered three paths for connecting the electrodes to the circuit to reduce the risk of cross-interference. It was shown that although the use of a large number of switches reduced noise, this greatly affected the complexity of the circuit, so the optimal wiring strategy was to use only one (1) switch.
An expected study question that was subsequently answered was how to determine the independence of the sensors from each other. Thus, the authors did a parallel experiment to measure two solutions, PBS and potassium hexacyanoferrate, and it was shown that each of the two sensors recorded the signal independently without any significant interference. The same was true for a solution containing different concentrations of metal salt. Although some noise was noticed, the authors were able to explain their nature by the equivalent connections of the elements in the electrical circuit. In addition, using multichannel amperometry, it is possible to measure the amount (or concentration) of breads in the ELISA experiment since it was shown that the peak current correlates with this number.
Pharmacological Research
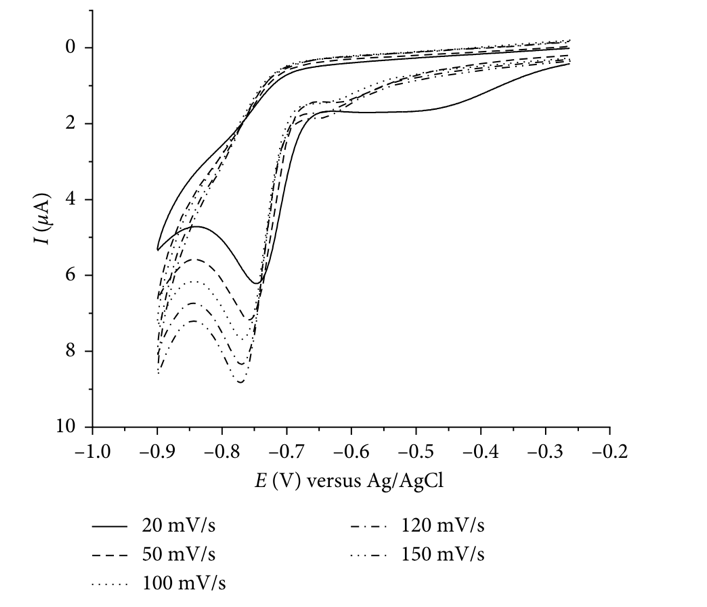
The signals measured by multipulse scanning technology testified to irreversible electrode reduction processes. Thus, when the scanning speed increased (from 20 to 150 mV s-1), there was a potential peak change to lower current values. In other words, the faster the scan was carried out, the less electricity output was recorded (Figure 4). In addition, the influence of the pH medium was determined in two directions at once:
- increased acidity of the medium moved the cathode peak towards more positive values, and
- alkalization of the medium worsened the signal. It follows that the reduction peak depends on the composite structure of the solution, but an infinite pH increase was not acceptable due to a decrease in the analysis quality.
Although the simultaneous measurements — such as analysis of the proposed scheme optimization models or calculation of analytical parameters — were made by Wua et al., the core of the study was the detection of diazepam in beverage samples. It was shown that even a small volume (0.05 ml) of drinks, guarana and two beer samples, was sufficient to quickly and accurately determine drug concentration. Thus, multipulse amperometry is a sufficiently accurate and highly sensitive measurement method.
Application of Amperometric Systems in Neurobiology
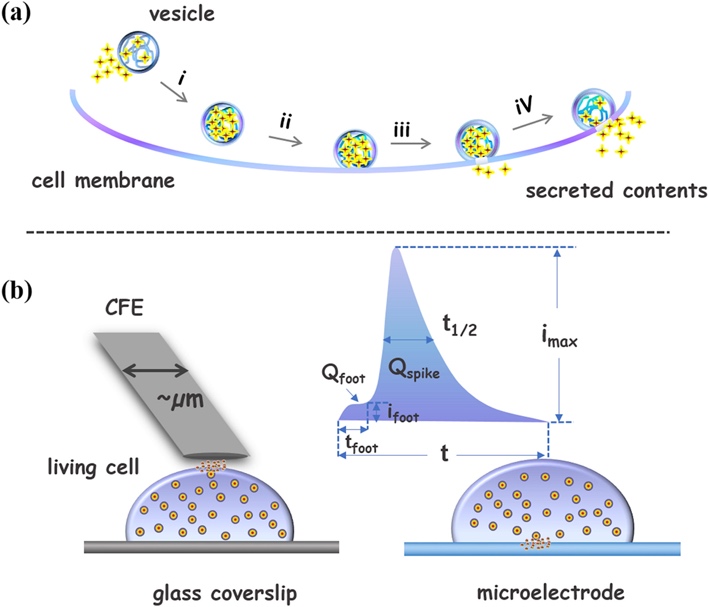
A few words should be said about the possibility of using the amperometric measurement in biological processes, and in particular in nervous tissue. Standard vesicular exocytosis is a biological process that occurs in the cells of plants and animals, which involves the movement of substances from the cell to the external environment. This type of isolation is an excellent strategy for intercellular communication, including the cells of human nervous tissue. Neuromediators — be it dopamine, serotonin, acetylcholine, or others — traditionally act as products of secretion of such cells, providing the transfer of nerve impulses in the gray matter of the brain.
According to Liu et al., amperometry is not a new method in studying activity in synaptic spaces, but previous attempts were limited by the received signals’ linearity (2019). Their study allowed expanding the scope of the analysis by providing a spatial estimation of diffusion current in neuro processes. In particular, the authors have shown that the use of glassy carbon microelectrode allows estimating the nature of the diffuse current emission at the contact of the vesicle with the membrane cell. The chief result of the author can be considered the discovery of the fact that the neurotransmitter transfer between neurons is a nonlinear and short-term process, which quickly produces a significant amount of electricity, as shown in Figure 5. Based on these data, it can be argued that the use of microelectrodes as an application of amperometry in neurobiological purposes is a promising practice.
Deficiencies of Amperometry
Despite all the achievements of amperometric application in the described cases, this method remains imperfect. First of all, it concerns the purity of the received signal: too many factors affect the final current strength. For instance, if there is an additional substance in the solution that reacts with an analyte or electrode material, it will affect the result. This is especially true for co-precipitation reactions observed in natural aquatic ecosystems. In addition, in some cases, dissolved oxygen must be removed to obtain more accurate results. The range of permissible voltage per electrode is also limited, as too high a value will cause unwanted chemical destruction. Furthermore, there is a limitation on the amount of foreign matter: its concentration must be lower than the concentration of the compound being measured. Finally, the selection of an electrode for a specific task can be a complex procedure that requires consideration not only of the chemical interaction between the plate wall and the solutions but also of the generated electrical field affecting the result.
Conclusion
During this work, it has been repeatedly noticed that classical amperometry has long ceased to be a conservative method of chemical analysis and has acquired the status of a flexible and controlled area of chemistry, which is used in interdisciplinary research. It is worth recalling that amperometry is the measurement of current strength (I) in any chemical process that results in the emission of a diffusion current. In this case, as in most electrochemical methods of analysis, there is a direct proportionality between the two parameters, current strength, and concentration of the substance. Thus, an increase in the current value can signal an increase in the concentration of the substance.
Exactly these properties were used in all considered scientific works: however, the research interest was caused not by the use of known methods but by their modification. The authors of each of the selected papers have concluded that it is acceptable to use modified electrochemical systems — whether fermented electrode, multiplexed signal acquisition system, or multipulse quantitative estimation — in the assigned tasks. In this case, summarizing the data from all materials, amperometry becomes a promising application for narrower areas.
Among others, amperometric research can be used to determine drug concentrations, assess signal densities, or detect the presence of heavy metals in water. Another great application of amperometry is the measurement of electrochemical reactions at the micro-level: the transfer of the neurotransmitter between neurons. Thus, amperometry is an incredibly important and valuable science with the potential to improve the knowledge of humanity in a wide variety of disciplines.
References
Amine, A.; Mohammadi, H. Amperometry. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier Inc. SciTech Connect: Cambridge, 2018; pp 1-13.
Antunović, V.; Tešanović, S.; Perušković, D.; etc. Development of a Flow Injection System for Differential Pulse Amperometry and its Application for Diazepam Determination. Journal of Analytical Methods in Chemistry, 2018, 6121489, 1-6.
Dalkıran, B. Amperometric Determination of Heavy Metal Using an HRP Inhibition Biosensor Based on ITO Nanoparticles-Ruthenium (III) Hexamine Trichloride Composite: Central Composite Design Optimization. Bioelectrochemistry 2020, 135, 1-12.
Liu, X.; Tong, Y.; Fang, P. Recent Development in Amperometric Measurements of Vesicular Exocytosis. Trends in Analytical Chemistry, 2019, 113, 13-24.
Wua, D; Rios-Aguirreb, D.; Chounlakoneb, M; etc. Sequentially Multiplexed Amperometry for Electrochemical Biosensors. Biosensors and Bioelectronics, 2018, 117, 522-529.