- Objective
- Synthetic Equation of the Reaction
- Physical Properties of the Reactants used and Products Generated
- Procedure Performed in the Lab
- Calculation of Theoretical and Percentage Yield
- A Short Synopsis of the Results Obtained during the Experiment
- A Discussion of the Results and the Outcomes of the Experiment
- Works Cited
Objective
The purpose of this experiment is to use the researcher’s background knowledge on alcohol reactions to explain more complex reactions. In addition, the test aims at reorganizing vicinal diol through the migration of a methyl group. The objective of this reorganization is to generate a ketone.
Synthetic Equation of the Reaction
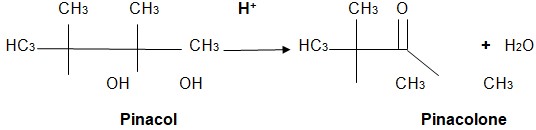
Below is the synthetic equation of the reaction:
Physical Properties of the Reactants used and Products Generated
Pinacol
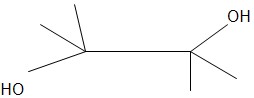
- Molar mass = 118.17 g/mol
- Density = 0.967 g/cm3
- Boiling Point = 171 to 173 °C
- Appearance = white solid
- Main hazard = irritation, explosion
Sulfuric Acid
- Molar mass = 98.079
- Density = 1.84 g/cm3
- Melting point = 10°C
- Boiling point = 337 °C
- Appearance = clear, colorless liquid
- Main hazard = irritant, toxic, flammable
Water
- Molar mass = 18.01528 g/mol
- Melting point = 0°C or 32°F
- Density = 1000 kg/m3
- Boiling point = 100°C or 212°F
Sodium sulfate
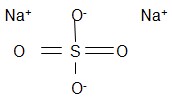
- Molar mass = 142.04 g/mol anhydrous and 322.20 g/mol decahydrate
- Melting point = 884°C or 1623 °F
- Density = 2.664 g/cm3 anhydrous and 1.464 g/cm3 decahydrate
- Boiling point = 1429 °C or 2604 °F
- Appearance = white crystal solid
- Main hazard = irritant
Sodium Chloride
- Molar mass = 58.44 g mol-1
- Density = 2.165 g/cm3
- Boiling point =1413 °C or 2575°F
- Melting point = 801°C or 1474°F
- Appearance = colorless crystals
- Main hazard = irritant
Pinacolone
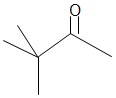
- Molar mass = 100.16 gmol-1
- Melting point = -52 °C or -62 °F
- Boiling point = 103 to 106°C or 217 to 223 °F
- Density = 0.801 g cm-3
- Appearance = colorless liquid
- Main hazard = irritant, flammable
2,4 – dinitrophenylhydrazine (2,4- DNP)
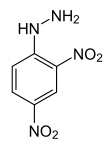
- Molecular mass = 198.14 g/mol
- Melting point = 198 to 202°C or 388 to 396 °F
- Appearance = orange or red powder
- Main hazard = carcinogenic, flammable
Procedure Performed in the Lab
A number of steps were involved in this experiment. To start with, 3.0 g of pinacol and 30 ml of deionized water were added into a 50ml round-bottomed flask. The mixture was then heat gently in a heating mantle until it dissolved. Once heated, the flask and its contents were cooled to room temperature. The cooling was achieved by dipping the flask in a bath of tap water. After this, 1-2 boiling chips were added to the solution. Then 5.0 ml of sulfuric acid was added. The acid was introduced slowly, carefully, and while swirling the solution. The mixture produced an exothermic reaction. When the flask seemed to be getting too hot, it was cooled in a bath of tap water. The concoction was swirled for ten minutes.
The next step involved connecting the round-bottomed flask to a simple distillation apparatus. The contents were then distilled into a 50ml round-bottom flask. The distillation process was stopped when the temperature of the vapor reached 100°C. After this, 5ml of saturated sodium chloride solution was added to the distillate and mixed well. The aqueous layer was then removed using a transfer pipette. However, it is important to note that the layer was not discarded. The reason for this is because nothing is disposed of until the experimenter gets a verification of IR. The organic layer was dried over anhydrous sodium sulfate and pipette. On its part, the dried liquid was decanted into a clean, dry, pre-weighed sample vial. The liquid was weighed and the percentage yield of the product calculated. Finally, IR and 2,4 – DNP tests were carried out on the product. The solution was then capped until the next lab session when NRM spectrum of the product was run.
Calculation of Theoretical and Percentage Yield
The computations below were used to determine the theoretical and percentage yield of the reactants and products in the lab experiment:
Theoretical Yield
- Mass of pinacol = 3.0g
- Moles of pinacole = 3.0g/ 118.17 g/mol = 0.02538 mol
- Theoretical yield = (0.02538mol*100.16g/mol)/ 1 mol of pinacolone = 2.542g
Percentage Yield
- Empty sample vial= 17.48g
- Sample vial + content = 18.87g
- Mass of product obtained: 1.39g
- Percent yield = (Actual/theoretical)*100 = (1.39g/2.542g)*100 = 54.68%
A Short Synopsis of the Results Obtained during the Experiment
The product (pinacolone) was obtained after the rearrangement of pinacole. After calculations were carried out, it was found that the product weighed 0.905g. However, the calculated theoretical yield was 1.39g. The pinacolone product showed a percentage yield of 54.68%. The results show that the product obtained was of substantial quantity. The reason behind this is because it was above 50%. The yield is an indication that a significant amount of the pinacol compound had been converted into pinacolone. Only a small percentage remained as the reactant. In the IR and NMR table, the IR spectrum reveals that there was a peak around 1451.28 -2942.41 cm-1 (-OH) stretch. The observation shows that there are minimal extra pinacols. Another reason associated with the high percentage yield is that most of the pinacol compound was utilized and did not evaporate during the distillation process. During such experiments, evaporation is caused by the high temperatures used to heat the compound. When there is too much evaporation, the number of pinacol molecules to be rearranged into pinacolone decreases. As a result, some experiments yield low levels of pinacolone product.
A Discussion of the Results and the Outcomes of the Experiment
When carrying out a pinacol rearrangement experiment, a number of factors must be taken into consideration. For example, the experimenter should note the hydroxyl group that is lost from the process as H2O. In addition, the carbocation that is steadier should be identified. The intrinsic changing trends of different substituent groups should also be taken into consideration during the experiment. Finally, the experimenter should note the influence of steric impediment on the rearrangement of the compounds. Other factors to be taken into consideration include whether or not the constancy of the products dictates the upshot of competing rearrangements. It should also be noted that the reaction conditions influence the course of the rearrangement during the experiment. Lastly, the experimenter should note whether or not epixodes are created as intermediaries in the pinacol experiment.
The pinacol – Pianacolone rearrangement is a procedure that involves turning a 1,2-diol into a carbonyl compound. The 1,2 – diol rearrangement process occurs under acid conditions. The experiment gets its name from the starting material, which is pinacol. The material is rearranged to pinacolone in the process. The reorganization allows a carbonyl group to be synthesized out of a diol. The synthesis takes place through a SN1 reaction mechanism. During the experiment, protonation of one of the OH groups takes place. Consequently, carbocation is created. In instances where the two OH groups are different, the one which produces a steadier carbocation is used in the reaction.
Alcohols are considered to be bad leaving groups. As a result, the reaction is an acid-catalyzed process. In the current experiment, concentrated Sulfuric acid (H2SO4) was used as a catalyst. It was used to protonate one of the alcohol groups to form a good leaving group. However, it is important to note that other acids can be used in place of sulfuric acid. The substitutes include perchloric, phosphoric, tosic, and triflouroacetic acids. However, concentrated sulfuric acid is the most preferred catalyst. The reason is because it minimizes dehydration during the experiment. The process is termed as an exothermic reaction. The round-bottom flask was cooled down using a bath of tap water instead of an ice bucket. The primary reason for this was to prevent re-crystallization. Protonation of the oxygen in the hydroxyl group allowed it to give a pair of electrons to the H+ and to create new oxygen-to-hydrogen bonds. The process converts the alcohol into an oxonium species (-OH2+). The conversion of alcohol into oxonium in the reaction takes place in relation to the standard migratory aptitude. In most cases, the oxonium ion has an absolute octet pattern at all centers. The oxonium is considered to be a good leaving group.
Loss of water gives a tertiary carbocation that is stable. The relatively steady tertiary carbocation can be converted into an even more stable carbocation. The enhancement can be achieved through the migration of a methyl group (methyl shift). The extra stability results from the process of placing the positive charge on the carbon atom where the second (-OH) group is located. The oxygen’s nonbonding electrons on the hydroxyl group are vital to the stabilization of the positive charge via resonance. Pinacolone, which is the product of the reaction, is generated after deprotonation of the resonance-stabilized cat ion.
The product has a carbonyl group. The group makes it different from the pinacole. Another important product used in the reaction is water. Pinacolone and water are co-distilled using a simple distillation apparatus. The aim is to detach the products of the reaction. The primary reason why water and pinacolone are important in the experiment is that the two are immiscible. The relationship can be explained using the Raoult’s law. According to this law, immiscible liquids are co-distilled below the boiling point of either component. The boiling point of water is 100 °C. As a result, no further distillation should take place after this degree is attained. The reason for this is because it is not certain whether or not the pinacolone has been sufficiently co-distilled with water. In addition, extra heating of the mixture will distill the unreacted pinacole. If the unreacted product is distilled, the correct outcome may not be achieved. In the current experiment, sodium chloride was added to the distillate to remove inorganic impurities and excess water. It was also used to further separate the two layers.
It is also noted that water and pinacolone have different densities. As a result, two distinct layers should be obtained after the distillation process is complete. The upper layer is often the organic pinacolone. The reason why it is the first is because it has a low density (0.801 g/mL) compared to the aqueous water layer (0.997 g/mL). Water should be removed from the final product. The major reason for this is to avoid interfering with the IR and the NMR spectra analyses. Anhydrous sodium sulfate was added to the organic layer during this experiment. The acid was used to ensure that all water molecules had been removed from the organic layer.
Three different sets of tests were carried out to make sure that pinacolone was generated. The IR spectrum underscores the importance of this reaction. It shows how the pinacole was converted into pinacolone. The pinacole spectrum shows a (-OH) stretch around 1451.28 – 2942.41cm-1. There is a peak shown on the IR of the pinacole. The peak illustrates the availability of the hydroxyl groups. On its part, the pinacolone spectrum does not indicate any (-OH) stretch. However, it contains a carbonyl (C=O) stretch around 3500-4000 cm-1. The stretch shows that a ketone has been synthesized as a result of pinacol rearrangement.
The NMR spectrum of the product (pinacolone) shows two peaks. The presence of these peaks illustrates that there are two forms of protons in it. They include the ones on the methyl groups and the ones attached to the tert-butyl. The peak that depicts the protons on the tert-butyl group is higher than the one showing methyl groups. The reason for this is because there are nine protons on the tert-butyl. On the other hand, there are three hydrogens on the methyl group. Both the tert-butyl and methyl peaks are singlet. The reason is because the methyl protons are next to a carbonyl group that does not contain any proton on it. In addition, the protons on the tert-butyl group are attached to a carbon that has no protons on it. The same case applies to the pinacole. The reason behind this is the number of splits, peaks, and differences in the sizes of the peaks. The only difference depicted in the pinacole spectrum is the peak on 2.3 ppm. The peak was seen to shift to the left. It shows that the protons directly attached to oxygen molecules in the pinacol are down-shielded. There is no shift in the spectrum of the product. The observation indicates that pinacolone was generated.
A 2,4-DNP test was also used to show that pinacolone, which has a carbonyl group, was produced after the pinacol rearrangement process took place. Color changes were also observed during the course of the experiment. After adding a few drops of the product to the 2,4-DNP reagent, an orange solid was formed. The orange compound is an indication of the presence of a carbon-oxygen double bond in the ketone (pinacolone). The 2,4-DNP reagent reacts readily with aldehydes or ketones. It achieves this through a condensation reaction. The isolated pair of electrons on the terminal amino group in 2,4-DNP reagent makes it a good nucleophile. The nucleophilic 2,4-DNP attacks the electrophilic carbonyl group of the pinacolone. As a result, it generates the orange colored compound, 2,4-dinitrophenylhydrazone. The figure below shows the pinacol mechanism.
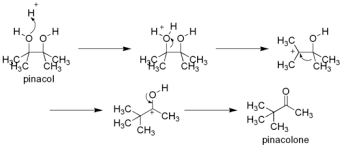
Pinacol rearrangement is regioselective. Due to this, the primary product is obtained from the rearrangement of the more stable carbocation.
Works Cited
Blanc, Guilaine. New Strategies for Pinacol Cross-Couplings and Alkenation Reactions, New York: Free Press, 2010. Print.
Gamini, Gunawardena, Pinacol Rearrangement. 2015. Web.
Klein, David. Organic Chemistry, Hoboken, NJ: John Wiley, 2012. Print.
McMurry, John. Fundamentals of Organic Chemistry, Belmont, CA: Brooks/Cole, 2011. Print.
Organic Synthesis: Pinacol Rearrangement. 2013. Web.
Rearrangements Induced by Cationic or Electron Deficient Sites2013. Web.