Objectives
- Convert pinacol into pinacolone using the technique of pinacol rearrangement, which entails dehydration of the pinacol by concentrated sulfuric acid and the rearrangement of the molecular structure by the methyl shift.
- Undertake qualitative analysis of the product (pinacolone) using IR and NMR techniques, which provide spectra of functional groups and chemical shifts respectively.
- Carry out qualitative analysis of the product using 2,4-DNP test, which can detect carbonyl groups in ketones and aldehydes.
Chemical Reactions
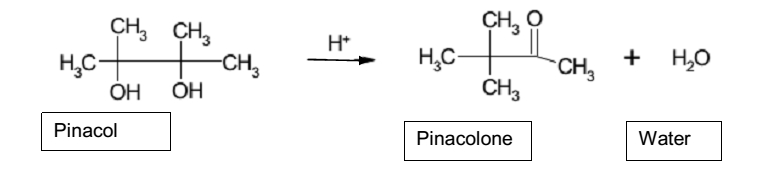
Pinacol rearrangement
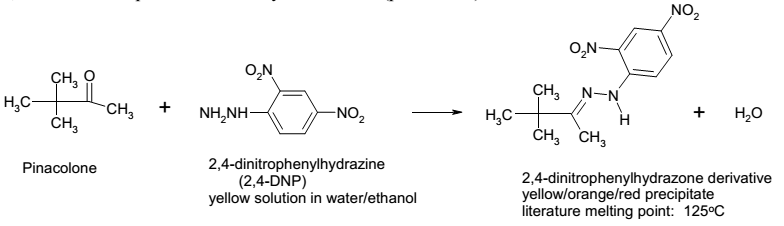
2,4-DNP test
Physical Properties
Pinacol
- State: Solid
- Pinacol Molecular formula: C6H14O2
- Molecular weight: 118.17 g/mol
- Water solubility: soluble in hot water
- Density: 0.967 g/ml
- Melting point: 40-43°C
- Boiling point: 171-172°C
- Hazards: Irritant and highly flammable
Sulfuric acid
- Sulfuric acid State: Liquid
- Molecular formula: H2SO4
- Molecular weight: 98.08 g/mol
- Water solubility: Miscible
- Density: 1.84 g/ml
- Melting point: 10°C
- Boiling point: 290°C
- Hazards: Highly flammable, corrosive, irritant, and toxic
Sodium Chloride
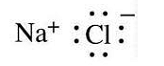
- State: Solid
- Molecular formula: NaCl
- Molecular weight: 58.44 g/mol
- Water solubility: 360 g/L
- Sodium Chloride Density: 1.199 g/ml
- Melting point: 801°C
- Boiling point: 1,413°C
- Hazards: Irritant
Sodium Sulfate
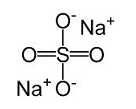
- State: Solid
- Molecular formula: Na2SO4
- Sodium sulfate Molecular weight: 142.04 g/mol
- Water solubility: 18.5 mg/L
- Density: 2.68 g/ml
- Melting point: 884°C
- Boiling point: 1700°C
- Hazards: Irritant
Water
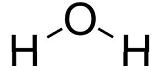
- State: Liquid
- Molecular formula: H2O
- Molecular weight: 18.015 g/mol
- Density: 1 g/ml
- Water Melting point: 0°C
- Boiling point: 100°C
- Hazards: Corrosive
Pinacolone
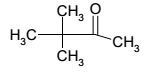
- State: Liquid
- Molecular formula: C6H12O
- Molecular weight: 100.16/mol
- Pinacolone Water solubility: 2.44 g/100 mL
- Density: 0.801g/ml
- Melting point: -52.5°C
- Boiling point: 106°C
- Hazards: Irritant, harmful, and highly flammable
2,4-DNP
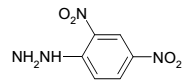
- 2,4-DNP State: Solid
- Molecular formula: C6H4N2O5
- Molecular weight: 184.11 g/mol
- Water solubility: 0.6 g/100 mL
- Density: 1,683 g/ml
- Melting point: 108-112°C
- Boiling point: 378°C
- Hazards: Highly flammable, toxic, harmful, and irritant, (“Chemical Book: Product Chemical Properties” par. 2)
Procedure
Pinacol rearrangement
To commence the experiment, 3 g of pinacol and 30 ml of deionized water were measured and put together in a 50 ml round-bottom flask to mix. The mixture in the flask was heated gently in a heating mantle until pinacol dissolved completely. The solution obtained was then cooled to room temperature by immersing the flask in a tap water bath.
Two boiling chips were added, and subsequently, 5 ml of concentrated sulfuric acid was added slowly and cautiously while swirling the contents of the flask for 10 minutes. Since the reaction between sulfuric acid and pinacol is an exothermic reaction, the flask was immersed in tap water bath to cool. The flask was then connected to a simple distillation apparatus and allowed to distill into another 50 ml round-bottom flask. The distillation process was stopped once the temperature of the vapors reached 100°C.
Next, 5 ml of saturated sodium chloride solution was added to the distillate in the flask and mixed thoroughly. The aqueous layer was removed from the flask using transfer pipette and placed in a beaker. The remaining organic layer was dried over anhydrous sodium sulfate and decanted, as the product of the experiment, into a pre-weighed sample vial that is clean and dry. The product was then weighed, recorded, and the percent yield of the product calculated. The IR and NMR analysis were performed on the product. Moreover, the 2,4-DNP test was performed to determine if the product formed is pinacolone.
The 2,4-DNP test
1 ml of 2,4-DNP reagent was measured using calibrated transfer pipette and put in a dry test tube. Subsequently, 5 drops of the product were added and mixed well with a stirring rod. Observation of the color changes and precipitate formation was made.
The Calculation of Theoretical Yield and Percent Yield
- Theoretical yield:
- Mass of pinacol = 3.0 g
- Moles of pinacol = 3.0 g/ 118.17 g/mol = 0.02538 mol
- Theoretical yield = (0.02538 mol*100.16 g/mol)/ 1 mol of pinacolone = 2.542g
- Percent yield:
- Empty sample vial = 17.48 g
- Sample vial + content = 18.87g
- Actual yield =18.87-17.48= 1.39 g
- Percent yield= (Actual/theoretical)*100= (1.39g/2.542 g)*100= 54.68%
Summary of Results
The experiment demonstrated the formation of pinacolone through the process of pinacol rearrangement. Theoretically, 3 g of pinacol, which is 0.02538 moles, would form 2.542 g of pinacolone. With 3 g of pinacol, the experiment formed 1.39 g of pinacolone, which constitutes 54.68% of the theoretical yield. The low yield of pinacolone implies that the experiment had some faults.
Although the pinacol was a limiting reagent, incomplete conversion into pinacolone might be responsible for the low yield. As the procedure of forming pinacolone entailed simple distillation, some pinacolone might not have been distilled or escaped the distillation apparatus. Another reason for low yield is that the loss of pinacolone through the decanted aqueous layer.
Qualitative analysis using the 2,4-DNP test generated a yellow precipitated, therefore, confirming that the product formed is pinacolone. Analysis of the IR spectra shows that the reactant has O-H stretch at 3440.25 cm-1 while the product has no O-H stretch around 3200-3600 cm-1. The absence of O-H stretch in the product means that pinacolone is pure without traces of pinacol. Moreover, the IR spectrum shows that the product has O=H stretch as 17.05.45, which indicates the presence of carbonyl groups. Hence, the IR spectra confirm that the product is pinacolone.
Additionally, NMR spectra of reactant and product depict different chemical shifts. The distribution of peaks reflects the distribution of protons in the pinacol and pinacolone. The depiction of unique chemical shifts implies that the pinacol rearrangement occurred.
Discussion
Pinacol rearrangement is a critical reaction of alcohols in organic chemistry that follows SN1 mechanism. Two important process of pinacol rearrangement is the dehydration and methyl shift. Dehydration process occurs when hydrogen ions from concentrated sulfuric acid protonate –OH by binding to the lone pairs of electrons in one of the –OH on the pinacol leading to the formation of oxonium ion (Anslyn and Dougherty 675). The ion weakens O-H bond and causes its cleavage resulting in the formation of oxonium ion, which is a good leaving group. The cleavage of the oxonium ion constitutes dehydration of pinacol because it leaves as a water molecule.
Given that the dehydration process causes the formation of the tertiary carbocation, it triggers methyl shift from the primary to the tertiary position. Essentially, the methyl shift allows the formation of carbonyl group resulting in the pinacol rearrangement. The reaction mechanism below illustrates the mechanism of pinacol rearrangement.
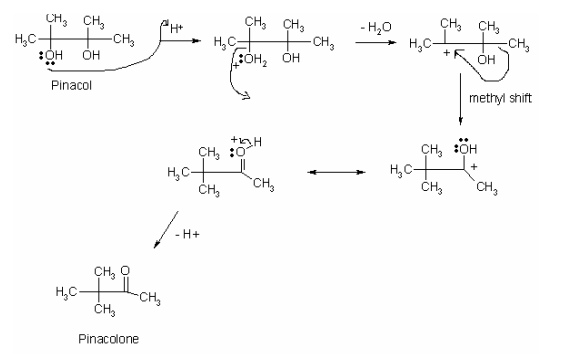
Sulfuric acid is an essential reactant in the reaction of pinacol rearrangement for it gives hydrogen ions that are necessary for the protonation of –OH group of pinacol. For carbocation to form, hydrogen ions must protonate –OH. Fundamentally, the carbocation that forms during pinacol rearrangement is significant in the SN1 mechanism. In the SN1 reactions, leaving groups determine the occurrence and the rate of a reaction. The –OH in pinacol is a bad leaving group because it binds tightly onto the carbon atom.
Hence, sulfuric acid makes –OH a good leaving group by protonating it and weakening its bond (Anslyn and Dougherty 675). When oxonium ion leaves pinacol, it creates a tertiary carbocation and forms water. The formation of water shows that pinacol arrangement involves a dehydration step. Since the tertiary carbocation is very stable, methyl group migrates to it in a bid to enhance its stability. Thus, sulfuric acid is critical for the formation of carbocation and the migration of the methyl from the primary site to the tertiary position.
Primarily, pinacol rearrangement is an organic chemical reaction that has reactants and products. The reactants of the reaction are pinacol and sulfuric acid while the products are pinacolone and water. Pinacol is a 1,2-diol (glycol), which is alcohol, whereas pinacolone is a ketone. As the reaction between pinacol and sulfuric acid is exothermic, it needs cooling of the flask. In the experiment, the reacting contents were cooled down by immersing the flask in a tap water bath.
Water has a cooling effect on the flask because its temperature is considerably lower than that of the boiling contents of the flask. Water also has a high heat capacity, which makes it absorb a lot of heat with a slight change in temperature. The thermal conductivity of water allows it to disperse heat quickly to the surrounding water molecules. These properties of water make it a suitable coolant in the experiment.
A simple distillation technique was used to separate water and pinacolone from the mixture in the flask. Water and pinacolone have boiling points of 100°C and 106°C correspondingly, which are very close, thus impossible to separate them using a simple distillation technique. In this case, Raoult’s principle of distillation was applied in the co-distillation of water and pinacolone leaving other contents of the flask. Raoult’s law states that a mixture of two liquids with almost the same boiling points can co-distill at the same temperature, which is usually less than the boiling temperature of both liquids (Reger, Goode, and Ball 496).
In this view, the distillation process stopped when the temperature of the vapor reached 100°C because the boiling point of the mixture is lower than 100°C. Additionally, a temperature that is greater than 100°C could allow the distillation of other liquids, hence, contaminating the distillate.
The content of the flask has organic and aqueous phases. Water pinacolone forms the aqueous layer while pinacolone forms the organic layer. Saturated sodium chloride was added to remove a lot of water from the organic phase. Comparison of the densities shows that pinacolone has a density of 0.801 g/ml whereas water has a density of 1 g/ml. In this view, the densities indicate that the aqueous layer forms the lower layer while the organic layer forms the upper layer.
The results of the experiment indicate that the actual yield of pinacolone is less than the theoretical yield. The actual yield of pinacolone is 1.39 g while the theoretical yield is 2.542 g. The analysis reveals that the actual yield forms 54.68% of the theoretical yield. The possible experimental errors that contributed to the low yields of pinacolone are the inaccurate measurement of pinacol, poor pipetting of the aqueous layer, and the leakage of pinacolone during distillation. The qualitative analysis of the product using 2,4-DNP test gave a yellow precipitate. The 2,4-DNP test gives a positive test with solutions such as ketones and aldehydes, which have carbonyl groups. The formation of a yellow precipitate validated that the product tested is pinacolone because there are no other ketones or aldehydes in the experiment.
NMR spectra depict pinacol and pinacolone having different chemical shifts. The NMR spectrum of pinacol has three peaks, which indicate three types of protons. Pinacol has two tertiary –OH groups and four secondary –CH3 groups, which exhibit different chemical shifts.
From the IR spectrum, it is evident that peaks reflect the nature of protons that are in pinacol. Comparatively, pinacolone exhibits three peaks with different chemical shifts. Pinacolone is a ketone that has a secondary carbonyl group, three tertiary methyl groups, and a secondary methyl group. These three groups have different protons that give three peaks like the ones of pinacol, but they are more amplified according to their positions in the structure. Overall, NMR spectra analysis indicates that apparent chemical shift is due to pinacol rearrangement.
Additionally, IR spectral analysis shows that the reactant and the product exhibit a unique pattern of peaks and stretches. The reactant has both O-H and C-H stretches at 3440.25 cm-1 and 2984.99-2942.41 cm-1 respectively. The wide O-H stretch shows that the reactant is an alcohol while the C-H stretches show that it is an organic compound. In contrast, the product has no O-H stretch, but it has a prominent C=O stretch at 1705.45 cm-1, which confirms that the product is pinacolone. Therefore, IR spectra corroborate the 2,4-DNP test and NMR spectra in confirming that pinacol rearrangement occurred.
Works Cited
Anslyn, Eric, and Dennis Dougherty. Modern Physical Organic Chemistry. Sausalito, Calif: University Science Books, 2006. Print.
Chemical Book: Product Chemical Properties 2008. 2016.
Reger, Daniel, Scott Goode, and David Ball. Chemistry: Principles and Practice. Belmont: Brooks, Cengage Learning, 2010. Print.