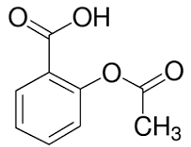
Aspirin
The chemical nature of aspirin is that it is acetylsalicylic acid (ASA), which is a synthetic derivative of salicylic acid. Aspirin is an analgesic that falls in the group of drugs called non-steroidal anti-inflammatory drugs (NSAIDs). Aspirin is a common analgesic that has numerous functions. The major functions of aspirin comprise the treatment of pain, inflammation, fever, stroke, blood clot, and heart attack. In its mechanism of action, Chan (2015) explains that aspirin irreversibly binds to cyclooxygenase, which is an enzyme that synthesizes thromboxanes and prostaglandins. Fundamentally, aspirin irreversibly inactivates cyclooxygenase by acetylating serine residue in its active site. The reduced synthesis of prostaglandins prevents the brain from receiving information of pain and inflammation while the reduced synthesis of thromboxanes hinders the formation of blood clots.
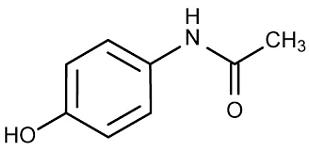
Acetaminophen
The chemical nature of Acetaminophen (Paracetamol) indicates that it is a synthetic and non-opioid analgesic derived from p-aminophenol. Acetaminophen is a mild analgesic because its major functions include treatment of pain and fever. It treats mild to moderate pain such as back pain, osteoarthritis pain, headache, postoperative pain, and cancer pain. Essentially, Acetaminophen is a mild analgesic because it has no anti-inflammatory activity. The mechanism of action reveals that Acetaminophen acts by selectively inhibiting cyclooxygenase-2, hence, preventing the synthesis of prostaglandins. Essentially, Acetaminophen binds to the active site of cyclooxygenase-2 and causes its inactivation (Hinz & Brune, 2014). Consequently, inactivated cyclooxygenase-2 fails to convert arachidonic acid into prostaglandin H2. The overall effect is that the concentration of prostaglandins decreases resulting in the inhibited transmission of pain and lowered fever.
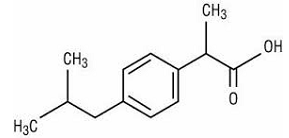
Ibuprofen
Ibuprofen is a synthetic analgesic, which is a derivative of isobutylphenylpropanoic acid and belongs to a group of drugs called non-steroidal anti-inflammatory drugs. As an analgesic, Ibuprofen relieves fever and pain and treats inflammation. The common uses of Ibuprofen are relieving pain associated with menstruation, rheumatoid arthritis, and migraines. Since Ibuprofen is a non-steroidal anti-inflammatory drug, it inactivates cyclooxygenase, which is an enzyme that synthesizes prostaglandins. It is a non-selective inhibitor of cyclooxygenase in that it inhibits both cyclooxygenase 1 and 2. Fundamentally, cyclooxygenase catalyzes the conversion of arachidonic acid to prostaglandin H2, which is a precursor to all other forms of prostaglandins (Bushra, & Aslam, 2010). Reduced concentration of prostaglandins inhibits transmission of pain while the reduced concentration of thromboxane prevents the formation of blood clot.
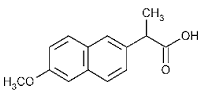
Naproxen
Naproxen is a synthetic analgesic that belongs to the group of non-steroidal anti-inflammatory drugs. The functions of Naproxen are relieving fever, pain, inflammation, stiffness, and swelling. In essence, Naproxen can treat conditions such as migraines, tendinitis, kidney stones, rheumatoid arthritis, gout, menstrual cramps, psoriatic arthritis, and dysmenorrhea. Given that Naproxen is a non-steroidal anti-inflammatory drug, it is a non-selective inhibitor of cyclooxygenase enzymes, namely, COX-1 and Cox-2. Naproxen reversibly binds to these enzymes and inactivates them, hence, preventing the synthesis of thromboxanes and prostaglandins (Husain, Yaseen, Rehman, Sarwar, & Tabish, 2013). The absence of thromboxanes eliminates the formation of blood clots. Moreover, the absence of prostaglandins prevents transmission of pain in the body.
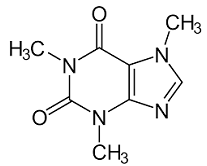
Caffeine
Caffeine is an alkaloid derived from plants as a potent analgesic and a stimulant. Chemically, Caffeine belongs to a class of chemicals derived from plants called methylxanthine. As a potent analgesic, Caffeine can relieve pain in conditions such as headache, back pain, arthritis, and migraines amongst others. Moreover, Caffeine is a common stimulant used in stimulating the central nervous system to increase physiological activities, enhance alertness, and improve coordination of the body. As an analgesic, Caffeine has antinociceptive effects because it inhibits adenosine a2a and a2b receptors resulting in reduced sensitivity to pain (Tavares & Sakata, 2012). Moreover, Caffeine relieves pain by reducing the activity and synthesis of cyclooxygenase.
References
Bushra, R., & Aslam, N. (2010). An Overview of Clinical Pharmacology of Ibuprofen. Oman Medical Journal, 25(3), 155-166.
Chan, A. (2015). Abstract CN05-02: Exploiting aspirin’s mechanism of action for combination chemoprevention. Cancer Prevention Research, 8(2), 1-23.
Hinz, B., & Brune, K. (2014). Paracetamol and cyclooxygenase inhibition: Is there a cause for concern? Annals of the Rheumatic Diseases, 71(1), 20-25.
Husain, M., Yaseen, Z., Rehman, S., Sarwar, T., & Tabish, M. (2013). Naproxen intercalates with DNA and causes photocleavage through ROS generation. The FEBS Journal, 280(24), 6569-6580.
Tavares, C., & Sakata, R. (2012). Caffeine in the Treatment of Pain. Rev Bras Anestesiol, 63(3), 387-401.