Introduction
A buffer is an aqueous solution that can neutralize small quantities of an acid or alkali, thus no significant change in overall pH. It contains either a weak acid and its conjugate base, or a weak base and its conjugate acid. Weak acids are slightly dissociated in dilute aqueous solutions. They produce small concentrations of hydroxonium ions and conjugate base when reacting with water. Thus, they react by replacing strong acids and bases with weaker ones maintaining the overall pH. The Henderson-Hasselbalch gives a range for buffer solution. The equation links the measured pH of a solution, hydrogen ions concentration, and the dissociation constant of the acid. It is also used to determine the pH of the buffer (Rilbe 34). The equation is as follows,
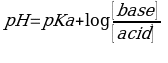
The equation is derived from weak acid dissociation equilibrium
- HA + H2O ↔ H3O+ + A–
It’s derived from a dissociation equation of a weak acid,
- Ka = [ H3O+][A–][HA–]
Taking –Log on both sides,
pH = -Log [ H3O+] = -log Ka-Log [ H3O+][A–]
P Ka = -Log Ka, thus
- pH = P Ka + Log [ A–][HA]
The Henderson-Hasselbalch equation shows that the P Ka is close to the PH of a buffer so that base of an acid is close to 1 (Petrucci et al. 18). Therefore, the addition of acid and bases will have more effect. This can be determined using titration and determining the equivalent point.
Procedure
The pH meter is calibrated with buffers of known pH. The pH meter is calibrated by pH 4 and 7 when examining acetic acid-acetate buffer and pH 7 and 10 for ammonia-ammonium buffer. 0.1 M acetic acid was added to sodium acetate and thoroughly mixed. The pH of 0.1 M acetic acid and acetate buffer solutions was measured. 0.1 M ammonia was added to 0.1 M ammonium chloride and stirred. The pH of 0.1 M ammonia and ammonium buffer solution was recorded. 1.0 M of sodium chloride and hydrochloric acid were prepared. Known volumes of sodium hydroxide and hydrochloric acid were added to both buffer systems each containing a different volume of buffer 1ml, 2ml, 3ml, and 4ml. The pH of each was recorded (Grossie 26).
Results and Calculation
Acetate buffer solution
Ka = [H+] [CH3COO–]
CH3COOH = (x) (1.0) 1.0
X =Ka =1.8 x10-5 = 4.74
Acetic acid
Ka= H+] [CH3COO–]
CH3COOH = (x) (x)
0.1 – x
X2/ (0.1-x) =1.8 x10-5
x = 1.3 x10-3
Ka = 2.9
Table 1: Calculated pH and measured pH of acetate buffer system.
Ammonia buffer solution
Kb= [NH4+] OH–]
NH3 = (x) (1.0)
1.0
X =Ka =1.8 x10-5
Kb = 4.74
Ka =14-4.74
Ka = 9.26
Ammonia solution
Kb= 4.74 + (-log 0.1) 2 = 2.87
Ka = 14 -2.87 =11.
Table 2: Calculated pH and measured pH of an ammonium buffer system.
Discussion
The pH of the buffer solution was measured using a pH meter and the calculated values were obtained from the Henderson-Hasselbalch equation. Hydrochloric acid and sodium hydroxide was used as strong acid and base respectively.
In the acid acetic acid and sodium acetate buffer system, when HCl was added, the C2H3O2– react with H3O+ according to the following equation.
C2H3O2– + H3O+ ↔ HC2H3O2 + H2O
When a sodium hydroxide was added to the buffer HC2H3O2 reacts to neutralize the OH– according to the following equation.
HC2H3O2 + OH– ↔ C2H3O2– + H2O
Table 1 and 2 above show both calculated and measured values of the pH after the addition of a strong base and a strong acid. The calculated values were very close to the measured values obtained using a pH meter. When a strong base or acid was added to the buffer solution, the pH increased or dropped respectively by a small margin.
The result of the experiment shows that the measure values were lower than the calculated values. Although, the overall change in the PH was very small. The differences in values may have resulted from the error during the preparation of the concentration standard in weighing the salts and adjusting the volume.
Henderson-Hasselbalch equation assumes that the concentration of the acid and base will remain the same by neglecting the dissociation of the acid. Also, it does not take into account the ionization of water. The equation also neglects the dissociation of acid and hydrolysis of a base. However, strong bases and acids which dissociate completely there were errors during the calculated values from the Henderson-Hasselbalch equation.
Conclusion
Experiment shows that buffer has resistance to change in pH on addition of an acid or a base. Buffer solutions are composed of both basic and acidic ions that are in a state of equilibrium. The PKa of the acids and bases for the calculated and measured value showed a slight difference. For the acidic buffer system, the calculated value was higher than the measured value. In the alkali buffer, the measured value was higher than the calculated value.
Works Cited
Grossie, David, and Kirby Underwood. Laboratory Guide for Chemistry.7th ed. 2014. Dayton: Wright State University. Print.
Petrucci, H. Ralph, William S. Harwood, Geoffrey F. Herring, Jeffry D. Madura. General Chemistry: Principles & Modern Applications. 9th ed. 2007. Upper Saddle River, New Jersey: Pearson/Prentice Hall. Print.
Rilbe, H. pH and buffer theory: a new approach. Chichester: Wiley, 1996. Print.