Introduction
Gluten is the protein component of the endosperm of cereal grains. The role of gluten in growing plants is to provide nourishment during the germination phase (Koehler & Wieser 2013). When cereal grains are ground, gluten affects the elasticity of the resulting flour, which is observed as chewiness in baked goods. Gluten consists of two types of proteins called gliadin and glutenin. Gliadin is a prolamin protein, whereas glutenin is a glutelin protein (Balakireva & Zamyatnin 2016).
Plants that contain gluten include wheat, barley, rye, and triticale. Baking flour is classified as soft, medium or hard depending on the gluten content. Hard flour contains the highest amount of gluten with a concentration of between 12 and 14%, whereas soft flour contains lower amounts of gluten than those in hard flour (King 2012). Therefore, the gluten content of flour affects the physical properties of flour and the corresponding baked products. Raising agents (also referred to as leavening agents) also affect the overall texture of baked goods.
Raising agents fall into three main categories: natural, mechanical, and chemical. Natural leavening may be achieved by the production of carbon dioxide gas through fermentation. Examples of natural leavening agents are eggs, yeast, steam, and dry heat. Mechanical leavening involves the inclusion of air into dough through physical means such as whipping of eggs or sifting of flour. Examples of chemical raising agents include sodium bicarbonate, cream of tartar, and baking powder. Sodium bicarbonate, which is frequently referred to as baking soda, is an alkaline substance that is commonly used to leaven soda bread and ginger bread.
Cream of tartar is an acid with several chemical names such as potassium hydrogen tartrate, potassium bitartrate, monopotassium tartrate, and potassium acid tartrate. Potassium bitartrate is usually used alongside baking soda to provide the acidity required to activate the baking soda. Baking powder is made by combining sodium bicarbonate and potassium hydrogen tartrate. The addition of water to baking powder sets off a reaction between the two components, which liberates carbon dioxide gas.
Factors affecting the activity of chemical raising agents include temperature and pH. An increase in temperature increases the release of carbon dioxide gas from the raising agent, thus leading to a corresponding increase in the volume of the baked product. Reducing the pH of the raising agent by adding an acid increases the action of chemical raising agents through the liberation of carbon dioxide gas. Consequently, too much alkalinity lowers the action of chemical raising agents. The purpose of this lab was to ascertain the impact of chemical leavening agents on flour mixtures.
Results
It was observed that the rising of bread was proportional to the amount of chemical raising agent. However, there was no significant increase in rising at concentrations beyond 100% of the raising agent. The findings are summarized in Table 1 and Figure 1. The addition of water to different raising agents elicited different reactions as summarized in Table 2.
Table 1: Effect of chemical raising agent on rising of bread.
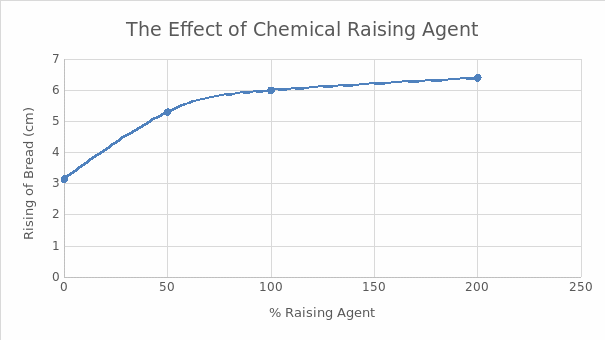
Table 2: Litmus paper test outcomes.
Discuprossion
The objective of the lab was to establish the influence of chemical raising agents on flour mixtures. In the first portion of the experiment, it was observed that increasing the concentration of the leavening agent led to a corresponding increase in the rising of bread (Table 1). The leavening agent was baking powder, which consists of sodium bicarbonate and potassium bitartrate. Adding water to the flour mixture containing the raising agent triggered a reaction between the acid (monopotassium tartrate) and base (sodium bicarbonate) to produce carbon dioxide and water. The carbon dioxide gas led to the rising of bread. Additionally, the water liberated during the process generated steam, which also contributed to leavening (Provost et al. 2016).
The gluten content of the flour played a significant role in the rising of bread. Gluten is reported to have exceptional water absorption and retention capabilities. Adding water to flour hydrates the gliadin and glutenin proteins, which combine to form gluten (Balakireva & Zamyatnin 2016). The addition of water to flour causes gluten to swell and create an incessant network of delicate strands. This network is what comprises the structure of bread dough and makes it flexible and stretchable.
The network of strands also entraps gas and contains it in the course of baking, which contributes to leavening. The overall outcome of rising in baked items is attributed to the extension of the gluten system. During kneading, mechanical forces cause stress to gluten and break the bonds between protein chains, thereby permitting the chains to move and realign. As the bread bakes, gluten coagulates in the new positions and stops being elastic. Coagulation determines the shape and size of the baked product and ensures that these properties do not reverse even after cooling.
It was noted that the bread still rose even without adding baking powder (Figure 1). However, the magnitude of the rising was smaller than when baking powder was used. This observation could be attributed to other leavening mechanisms such as the natural leavening from eggs and steam generated from the water portion of milk, which was part of the ingredients. Mechanical leavening through the incorporation of air by sifting also played an important role.
According to Pareyt et al. (2011), the volume of bread is influenced by the presence and composition of lipids. The source of lipids includes endogenous wheat lipids and exogenous sources (fats and shortening). Gliadin and glutenin interact with lipids through hydrophobic and hydrogen interactions (Pareyt et al. 2011). The binding of lipids to gluten allows the lipids to associate at the boundary of gas cells in the first stages of dough making. This process stabilizes the resultant gas cells that aerate bread (Verheyen et al. 2015). Lipids also enhance the inclusion of air into the dough in the course of mixing, which promotes the rising of dough.
The quality of gluten in dough contributes to the properties of bread. Poor quality gluten (low quantity) fails to stretch in thin films around air bubbles leading to the bursting and escaping of air bubbles. Consequently, the baked product lacks volume. On the other hand, too much of gluten hinders stretching, which limits the ability of gas bubbles to expand and facilitate the rising of the dough.
In the second part of the experiment, it was observed that there was no reaction when water was added to bicarbonate of soda (Table 2). This observation was attributed to the dissolution of sodium bicarbonate into sodium hydroxide and hydrogen carbonate. Sodium hydroxide is an alkaline compound, which turns red litmus paper to blue. This observation implies that sodium bicarbonate alone cannot be used effectively as a raising agent because it does not liberate carbon dioxide to raise dough in the absence of heat (Provost et al. 2016). However, when heated to high temperatures such as those present during baking, sodium bicarbonate decomposes to yield sodium carbonate, water, and carbon dioxide gas (Provost et al. 2016).
When water was added to cream of tartar, the compound dissolved slightly to form an acidic solution that turned blue litmus paper to red. This observation means that cream of tartar alone is not an effective raising agent.
However, cream of tartar can be used as a stabilizing agent in recipes that involve incorporation of air, for example, whipped cream and meringues (Pauling 2014). When water was added to baking powder, the observed effervescence was a result of escaping carbon dioxide gas, which was produced from the chemical reaction between sodium hydrogen carbonate and cream of tartar (the active ingredients of baking powder) (Provost et al. 2016). The implication of this reaction is that during baking, heat leads to further decomposition of sodium bicarbonate to liberate carbon dioxide, which leavens the baked product.
A similar reaction was observed when water was added to a blend of baking soda and potassium acid tartrate because the acid triggered the liberation of carbon dioxide gas. These observations mean that sodium bicarbonate can only be used effectively as a raising agent when an acid is a part of the ingredients. In the absence of potassium bitartrate, other acidic liquids such as vinegar, lemon juice, and sour milk can be used alongside baking soda. Additionally, dough made using this procedure needs to be baked immediately after preparation to ensure that the gas does not escape. There was additional effervescence when the components of tubes 3 and 4 were heated because heating led to the additional production of carbon dioxide gas.
The findings of this lab point to the realization that even though chemical raising agents facilitate the rising of baked goods, other mechanisms of raising also contribute to the final volume of the baked product. Consequently, it is important to follow the recipe accurately. Baking is a dynamic process, and the quality of the final product is influenced by an array of factors such as the quality of wheat flour, the quality and quantity of raising agent, as well as the dough preparation process.
Reference List
Balakireva, AV & Zamyatnin, AA 2016, ‘Properties of gluten intolerance: gluten structure, evolution, pathogenicity and detoxification capabilities,’ Nutrients, vol. 8, no. 10, p. 644.
Koehler, P & Wieser, H 2013, ‘Chemistry of cereal grains,’ in M Gobbetti & M Ganzle (eds), Handbook on sourdough biotechnology, Springer, USA, pp. 11-45.
Pareyt, B, Finnie, SM, Putseys, JA & Delcour, JA 2011, ‘Lipids in bread making: sources, interactions, and impact on bread quality,’ Journal of Cereal Science, vol. 54, no. 3, pp. 266-279.
Pauling, L 2014, General chemistry, WH Freeman, Courier Corporation.
Provost, JJ, Bodwin, J, Kelly, BS, Colabroy, KL & Wallert, MA 2016, The science of cooking: understanding the biology and chemistry behind food, John Wiley & Sons, Hoboken, NJ.
Verheyen, C, Albrecht, A, Elgeti, D, Jekle, M & Becker, T 2015, ‘Impact of gas formation kinetics on dough development and bread quality,’ Food Research International, vol. 76, pp. 860-866.