Abstract
Colorectal cancer (CRC) is a significant health concern in the United States, yet reluctance to screen remains high. At the project’s site, there was a need for an evidence-based model that can be implemented to improve screening rates. The purpose of this quantitative quasi-experimental quality improvement project was to determine if or to what degree the implementation of the Agency for Healthcare Research and Quality’s System Approach to Tracking and Increasing Screening for Public Health Improvement of Colorectal Cancer (AHRQ’s SATIS-PHI/CRC) toolkit would impact colon cancer screening rates when compared to current practice among patients aged 50-75 in a primary care clinic in urban New York, over four weeks. Hochbaum et al.’s health belief model (HBM) and Rogers’ protection motivation theory (PMT) were the theoretical underpinnings of the project.
Participants were 100 patients recruited through nonrandomized sampling from an urban Ney York clinic. The CRC screening rates of the sample were obtained from the electronic health record system and analyzed using the Chi-square test. The analysis of the impact of using the SATIS-PHI/CRC toolkit to redesign the screening process showed at X (1) = 88.360, P=.000, suggesting that there was a statistically significant effect of the intervention on screening rate. Further, the project’s results showed that the toolkit improved screening rates, and healthcare facilities should adopt the model. Researchers should conduct further research with larger sample sizes for future projects.
Introduction to the Project
At present, it is not yet determined whether redesigning the colorectal cancer (CRC) screening process can lead to increases in the number of patients undergoing screening compared to the interventions already in place. Even without clarity over the efficacy of early screening for CRC, there have been indications of a downward trend in CRC incidence rates. In particular, between 2013 and 2017, the disease incidence rate declined by1% every year over this period (American Cancer society, 2021a). However, despite the decline in CRC cases between 2013 and 2017, the focus is mainly on older adults. As a result, the true extent of the prevalence of this condition among younger adults is yet to be clearly understood (American Cancer Society, 2021a). When it comes to CRC screening, younger adults may not be receiving sufficient attention.
Older adults are especially vulnerable to CRC. With old age, individuals become susceptible to diseases that can reduce their lifespan. However, adherence to screening guidelines has been lax in the uptake of screening services. In 2010, for instance, only 59% of individuals aged 50 and above for whom screening was recommended had undergone screening (American Cancer Society, 2014). Screening has the potential to prevent CRC as it can detect precancerous growths, such as polyps, in both the colon and the rectum (American Cancer Society, 2014). While most polyps do not become cancerous, the American Cancer Society (2014) advises that the polyps be removed to reduce the chances of CRC (American Cancer Society, 2014).
The essence of early screening is to increase the opportunity for detecting CRC in its initial stages, thereby allowing medical professionals to devise measures to manage the disease. Thus, early screening for CRC is critical toward maintaining the well-being of older adults. It is true that there is already extensive research exploring CRC among older adults. However, the current project is still significant because it sheds further light on the role that CRC screening can play in preventing the development of this condition. More importantly, the project is important because it aimed to establish whether screening conducted using the SATIS-PHI/CRC is an effective intervention for increasing the rate of screening, thereby catching early cases of CRC in some instances while preventing CRC in others.
The purpose of this quantitative quasi-experimental quality improvement project was to determine if or to what degree the implementation of AHRQ’s SATIS-PHI/CRC toolkit would impact colon cancer screening rates when compared to current practice among patients aged 50-75 in a primary care clinic in urban New York over four weeks. Chapter One provides an overview of the introduction to the project and covers topics such as the project’s background, problem statement, the purpose of the project, the clinic question, and advancing scientific knowledge. Other issues that this chapter addresses include the significance of the project, rationale for the methodology, the nature of the project design, definition of terms, assumptions, limitations, and delimitations of the project, and the summary and organization of the remainder of the project.
Background of the Project
Medical screening remains one of the most effective measures to help identify and mitigate disease incidence and or continued progression following the adoption of early interventional measures. Retrospectively, the rate of individuals diagnosed with CRC annually has continued to drop since the mid-1980s (American Cancer Society, 2021a). The reason for this decline was that more people are undergoing screening, after which they adopted healthy behaviors, which have helped reduce their exposure to lifestyle-related diseases like CRC (American Cancer Society, 2021a). Therefore, the uptake of screening services proved sufficient to curtail the spread or rate of occurrence of CRC among the public. Nevertheless, the American Cancer Society (ACS) notes that CRC is the third most diagnosed cancer in women and men in the United States.
Furthermore, the American Cancer Society (2021a, 2021b) estimated that in this year, there are likely to be nearly104, 270 new cases of colon cancer and about 45,230 cases of rectal cancer. Such statistics underscore the need to devise measures that can help curb the spread of CRC. If left unattended, the prevalence of CRC will certainly present significant implications for the entire society. For example, the United States (U.S.) could continue to spend massive amounts every year to treat this condition.
The purpose of this quantitative quasi-experimental quality improvement project was to determine if or to what degree the implementation of AHRQ’s SATIS-PHI/CRC toolkit would impact colon cancer screening rates when compared to current practice among patients aged 50-75 in a primary care clinic, in urban New York, over four weeks. Essentially, the project sought to underscore the role of empirical data in informing the CRC prevention approaches that healthcare institutions put in place. Hence, this project was centered on predetermined criteria which limit participation to individuals aged 50 to 75 years old. The rationale was grounded on the understanding that older adults are highly susceptible to diseases, and preventive interventions like screening are highly recommended to maintain their health (Seematter-Bagnoud & Büla, 2018). Therefore, this project intended to find evidence that expressed the efficacy of early CRC screening using the SATIS-PHI/CRC toolkit in enhancing CRC screening rates.
Problem Statement
The American Cancer Society (ACS) (2021a) emphasizes that colorectal cancer (CRC) is the third most common cause of cancer death in women and men, and the second leading cause of cancer-related death in both women and men when combined in the United States (American Cancer Society, 2021a). The U.S. Preventive Services Task Force (USPSTF) (2016) previously recommended that adults aged 50–75 undergo CRC screening, with test options including fecal stool blood test yearly, flexible sigmoidoscopy every five years, or a colonoscopy every ten years. However, after the death of Black Panther actor Chadwick Boseman from CRC disease at the age of 42, proponents for early screening lobbied for change (Chiu, 2020). As a result, the USPSTF issued a new guideline on May 18, 2021, which lowered the recommended screening age from 50 to 45.
Today, the recommended age for CRC screening has dropped to 45 (Centers for Disease Control and Prevention, 2021b). Several scholars have stressed that routine screening and resultant early detection through a range of acceptable strategies (colonoscopy, fecal testing, among others) are helpful and cost-effective in reducing CRC incidence and mortality (Ran et al., 2019). Additionally, the ACS maintains a survival benefit to early detection: five-year survival for localized CRC is around 90%, which drops below 20% for patients diagnosed at late stages (ACS, 2020). Despite this information, screening rates remain relatively low; only 62.4% of age-eligible adults were up-to-date on CRC screening, falling short of national goals for CRC screening between 2000 and 2015 (White et al., 2017).
Further, minority race and ethnicity and socioeconomic challenges such as lack of insurance and low income are associated with higher CRC mortality (White et al., 2017). While there is an abundance of evidence that suggests that early screening saves lives, it is not known if, or to what degree, the implementation of the AHRQ’s SATIS-PHI/CRC toolkit would impact colon cancer screening rates when compared to current practice among patients aged 50-75. This population tends to be fearful of the process; they can better understand and feel confident about the screening procedure with education.
Purpose of the Project
The purpose of this quantitative quasi-experimental quality improvement project was to determine if, or to what degree, the implementation of AHRQ’s SATIS-PHI/CRC toolkit would impact colon cancer screening rates when compared to current practice among patients aged 50-75 in a primary care clinic, in urban New York, over four weeks. A quasi-experimental design was used alongside a quantitative approach to analyze the data. Further, White and Sabarwal (2014) explained that assignment to conditions is based on self-selection or administrator selection in a quasi-experiment. Therefore, this project looked to assess whether there had been any changes in patients’ screening status when comparing their state before and after the intervention. The project focused on patients within the age range of 50 -75 years. It took place in an urban primary care clinic in New York. Based on the clinical question, the variables were identified as the SATIS-PHI/CRC toolkit as the independent variable (IV) and screening rates as the dependent variable (DV). The project was intended to express the importance of preventive measures in fighting diseases.
Clinical Question(s)
The project was grounded on one clinical question: To what degree does the implementation of AHRQ’s SATIS-PHI/CRC toolkit impact colon cancer screening rates compared to current practice among patients aged 50-75 in a primary care clinic in urban New York? Based on the question given and using the AHRQ’s toolkit to redesign the screening process, a quantitative approach was used to determine the efficacy of the SATIS-PHI/CRC toolkit on screening rates within four weeks. The variables are colorectal cancer (CRC) screening using the SATIS-PHI/CRC as the independent variable (IV) and screening rate as the dependent variable (DV). The SATIS-PHI/CRC toolkit was used to guide the screening process.
Advancing Scientific Knowledge
The importance of this project was, in part, to advance the body of scientific knowledge. Currently, it is not yet determined whether early screening for colorectal cancer (CRC) using the SATIS-PHI/CRC toolkit can increase the rate of screening at an urban New York primary care clinic. The project was intended to provide evidence-based results that can be replicated or used to guide the adaption of health interventions when dealing with other diseases in other healthcare facilities, focusing on CRC prevention and treatment. Notably, the lack of evidence accenting the effect of early CRC screening using the SATIS-PHI/CRC on disease incidence highlights a gap within the medical community regarding the promotion of public health. Ebell et al. (2018) explained various barriers to adopting evidence-based medicine when providing treatment and care to patients. Ebell et al. (2018) made an important observation that medical education in the traditional context emphasizes pathophysiologic reasoning as the primary approach to treatment decisions. In the first two years, attention is given to the basic sciences. Such a mentality makes it difficult to adapt and apply new practices studied and proven effective through research.
However, despite the identified barrier, the project was geared toward providing more evidence about the importance of early CRC screening in curbing the spread and occurrence of CRC. The findings can be used to, for instance, help guide care providers when dealing with patients with other health conditions, which can affect the outcome of early CRC screening. Nevertheless, for those that already have mild symptoms of the disease, this project was intended to demonstrate whether, if exposed to early preventive measures, the occurrence of the disease can be studied effectively.
Therefore, this project was intended to add to existing scientific research regarding integrating and applying evidence-based medicine to address varied health problems such as CRC. The health belief model (HBM) is one of the theories that informed the project. The health belief model is a cognitive model extensively used to measure cancer screening beliefs and behaviors. It identifies patterns and barriers to healthy behaviors (Zare et al., 2016). The model was developed in the 1950s by social psychologists Hochbaum, Rosenstock, and others, working in the U.S. Public Health Service to explain the failure of people participating in programs to adopt health promotion solutions (Hochbaum et al., 1952). The model was later extended to study people’s behavioral reactions to health-related circumstances.
Rogers’s protection motivation theory focuses on health and describes one’s attitude and motivation to respond in a self-protective way to a perceived threat to one’s health. This theoretical model helps build self-efficacy and is beneficial in translating knowledge into healthy behaviors and lifestyles (Westcott et al., 2017). Alongside the health belief model discussed above, the protection motivation theory can encourage relevant and appropriate good health screening attitudes and behaviors. For instance, the two models could form the basis of encouraging those aged between 50 and 75 to undergo CRC screening.
Significance of the Project
CRC ranks third among the leading causes of death in America (American Cancer Society, 2021a). As a developed country, the U.S. stands to benefit from the already high-end health solutions at its disposal. For that reason, there is a need to promote the utilization of healthcare services through sensitization of the public regarding the importance of adapting health-seeking behavior. However, the uptake of preventive medicine is, in some cases, hindered because of the attitude and perception of patients. Scholars Alnaif and Alghanim (2009) noted that patients who lack sufficient knowledge about the various ways to safeguard themselves against diseases are unlikely to seek or utilize existing or recommended health services.
Thus, the disease incidence rate is likely to intensify, leading to the economic, social, and personal burdens on the patient and their family; and the healthcare system. Health literacy is a prerequisite to a healthy community. Therefore, there is a need to provide the public with access to necessary preventive medical services. The importance of health literacy explains Alnaif and Alghanim’s (2009) observation that health education is a cornerstone that helps manage chronic diseases such as asthma, obesity, hypertension, cardiovascular heart diseases, and others by providing patients with security and knowledge concerning their health. Through a change in attitude toward the importance of health services, individuals can change their behavior, enhancing their well-being while helping to secure public health. The project sought to demonstrate the efficacy of evidence-based medicine in promoting a healthy population. The results acquired will help guide the implementation of health intervention approaches. For instance, if early CRC screening proves effective, a similar approach can manage other chronic illnesses such as diabetes, asthma, and heart disease.
Rationale for Methodology
For the project, a quantitative approach was adopted. A quantitative methodology makes interpreting data and presenting the findings straightforward and less open to error and subjectivity (Devault, 2019). The structure of quantitative research allows for broader studies, which enables better accuracy when attempting to create generalizations about the subject matter involved (Gaille, 2019). Quantitative analysis utilizes mathematical, statistical, and computational tools to derive results. This structure makes conclusiveness to the investigated purposes as it quantifies problems to understand their prevalence (Gaille, 2019).
Moreover, the quantitative methodology uses homogenous steps to reduce or eliminate bias when collecting and analyzing data. This research approach is particularly advantageous for studies that involve numbers, such as measuring achievement gaps between different groups of patients or assessing the effectiveness of a new intervention (Dowd, 2018). Along with the quantitative method, the project incorporated a quasi-experimental approach to analyze the data acquired.
Nature of the Project Design
The project’s objective was primarily to determine the cause-and-effect relationship between the IV (early CRC screening using the SATIS-PHI/CRC toolkit) and DV (increased CRC screening rates). The project’s design followed a quasi-experimental approach. In support, White and Sabarwal (2014) explained that assignment to conditions is based on self-selection or administrator selection in a quasi-experiment. Therefore, the project looked to allocate participants in one group. The said group received pre and post-implementation education on early colorectal cancer screening using the SATIS-PHI/CRC intervention.
Participants were evaluated four weeks after implementing the said intervention to determine if there were changes in the rate of CRC screening. Because of the nature of the project, the quasi-experimental approach was deemed the best fit. It allowed the principal investigator (PI) to compare pre-implementation data and post-test results to determine the efficacy of the evidenced-based intervention on CRC screening. In part, the effectiveness of an investigation is determined by the validity and reliability of the findings. Additionally, higher external and internal validity justified the use of a quasi-experimental approach which took place in a natural setting. This made it less vulnerable to the manipulative nature of experiments in controlled environments (Reeves et al., 2017).
Compared to other designs, quasi-experimental designs are advantageous because they have a higher external validity as they involve real-world settings instead of artificial laboratory settings (Reeves et al., 2017). Secondly, there is a higher internal validity than non-experimental research because quasi-experimental studies allow the PI to control for confounding variables (Reeves et al., 2017). A confounding variable, such as the existence of co-morbid conditions, might affect the project’s outcome. For example, it could be that there is no change in symptomatology or risk of disease incidence for some patients who undergo early CRC screening because of the existence of another health condition. Here, disease co-morbidity is a confounding variable that is likely to make it difficult to determine the effect of the IV on the DV. Therefore, the quasi-experimental approach is ideal for determining whether early CRC screening will promote positive health outcomes in patients.
In the current case, the PI was able to observe and record the findings without any interference, thereby strengthening the legitimacy and increasing the potential applicability of the results. The sample involved 100 participants who were patients in the urban primary care clinic. At the close of the project, recorded data focused on the screening rates for CRC. In addition, pre and post-intervention data were compared to determine a statistically significant change in CRC screening rates.
Definition of Terms
The Definition of Terms section of Chapter One defined the project’s concepts. In addition, it provided a brief overview of the technical terms, exclusive jargon, concepts, and terminology used within the project’s scope. Terms are defined in layperson terms in the context in which they are used within the project. The following is a glossary of terms that were used recurrently in this project:
Colorectal cancer (CRC)
CRC refers to cancer in the colon or rectum (Centers for Disease Control and Prevention, 2020).
Disease Incidence
Disease incidence refers to the occurrence of new cases of disease (CDC, 2012).
Mortality
For this project, mortality refers to death rate or the number of deaths in a certain group of people in a given time period
Rectum
The rectum refers to the passageway that connects the colon to the anus (Centers for Disease Control and Prevention, 2021a).
The System Approach to Tracking and Increasing Screening for Public Health Improvement of Colorectal Cancer (SATIS-PHI/CRC) intervention
The SATIS-PHI/CRC toolkit refers to a population-based, system-level redesign of the way CRC screening and follow-up are conducted in a network of primary care practices (for example, in medicine clinics) (AHRQ, 2018a; Harris & Borsky, 2010).
Healthcare Effectiveness Data and Information Set (HEDIS)
HEDIS refers to a comprehensive set of standardized performance measures designed to provide purchasers and consumers with the information they need for a reliable comparison of health plan performance. In addition, HEDIS measures identify opportunities for improvement, monitor the success of quality improvement initiatives, track progress, and provide a set of measurement standards that allow comparison with other plans (Centers for Medicare & Medicaid Services, 2021).
Assumptions, Limitations, Delimitations
In this project, there are varied assumptions, limitations, and delimitations. The main premise was that this quality improvement project would accurately represent the current problem of low CRC screening rates in the New York hospital’s primary care clinic. For example, due to the current COVID-19 pandemic, patients have not been following up diligently. The hospital has noted the problem and has provided transportation services to transport some patients to and from their appointments and has enlisted the support of social workers and nurses in reaching patients to assess their needs and promote adherence with screening exams and following up appointments.
The SATIS-PHI/CRC intervention tool provides a mechanism for identifying patients eligible for but not up to date in their CRC screening. Additionally, the toolkit provided an avenue for contacting such patients on behalf of their physician’s practice to encourage and administer recommended screening, track screening results, facilitate patient notification, and appropriate follow-up through feedback to providers (Harris & Borsky, 2010).
It was assumed that all health care professionals participating in the implementation of the project would understand the applicability of the model and the benefits to patients and the facility arriving at the best possible outcomes for the patient. The goal was to improve the rates of CRC screening. Providers and other participants received education on how to use the tool. There was enough training to ensure a clear understanding of the tool. The PI addressed all questions and concerns pre-implementation as they arose. According to AHRQ, using academic detailing and performance feedback forms, the toolkit seeks to educate clinical providers, and other staff in participating practices about recommended CRC screening and follow-up procedures (AHRQ, 2018a).
Limitations of a project are any potential weaknesses or restrictions in a project that is primarily out of the investigator’s control and can affect the design and results or outcomes of the investigation (Ross & Bibler Zaidi, 2019). When it comes to limitations, the project was focused on individuals aged 50-75 years. While it is true that patients in this demographic group are most at risk for CRC, by focusing exclusively on this population, the project essentially disregarded younger patients for whom screening is now recommended. The issue here is that it would be rather challenging to apply the findings and conclusions from the project to a younger population, given that one’s age heavily influences their health outcomes.
For instance, younger adults are more vibrant and can engage in vigorous activities, which might undermine the ability to detect symptoms of CRC compared to older adults. However, to account for the effect of the limitations, the project was meant to increase knowledge regarding ways to improve screening rates and manage the spread and occurrence of CRC among the measured population. In doing so, the PI was able to find specific information that applied to the chosen targeted population.
Delimitations are the definitions one sets as the boundaries or limits of their project to so that the aims and objectives do not necome impossible to achieve. Delimitations are mainly concerned with the study’s theoretical background, objectives, research questions, variables under study and study sample (Thefanidis & Fountouki, 2019). The project was delimited to individuals who met all the criteria of the project. Therefore, individuals at risk for CRC and not at the required age were not selected as part of the population. Another delimitation for the project was that the participants were delimited to only one New York hospital primary care clinic, which accounts for just one city hospital in New York. However, 11 hospitals and multiple primary care clinics in the network face similar problems with CRC screening.
For example, two hospitals and their clinics work closely with the gastroenterologists practicing collaboratively and alternating days at both facilities. However, the patients seen in one hospital are not automatically seen in the other unless there is a referral from one physician or provider to the next. The providers go in both places, but the patients have to stay in one location, whichever one they choose. Therefore, the investigation findings can be generalized, and the project’s results can be applied to both facilities. Both facilities have a similar makeup of patients with identical poor or low screening rates problems.
Summary and Organization of the Remainder of the Project
The significance of CRC screening for adults aged 50 to 75 years has been well recognized and primarily accepted by the medical community. The American Cancer Society (ACS) emphasized that CRC is the third most common cancer and the second leading cause of cancer death in the United States (ACS, 2017). In 2017, the agency reported an estimated 95,520 new cases of colon cancer and 39,910 cases of rectal cancer diagnosed in the United States (ACS, 2017). The ACS projected that CRC would cause about 52,980 deaths during 2021 (ACS, 2021b). Despite the benefits of early cancer detection and the availability of practical screening tests, rates of CRC screening remain low (Modica et al., 2019). According to the ACS, in 2010, only 59% of those eligible for CRC screening received a screening test (ACS, 2014). Scholars stressed that routine screening and resultant early detection through a range of acceptable strategies (colonoscopy, fecal testing, among other test) are helpful and cost-effective in reducing CRC incidence and mortality (Ran et al., 2019).
Thus far, a review of the project’s background was provided, along with the clinical question upon which the project was grounded. A look at the background information highlighted the lack of evidence in determining the efficacy of early CRC using the SATIS-PHI/CRC toolkit on CRC screening rates. While the project focused on adults in the 50-75 age groups, it was intended to provide evidence that could be replicated and applied in other settings. In the next chapter, a discussion of existing literature on the medical issue at hand is provided. Theoretical frameworks are also highlighted to help offer a link between the project’s purpose, the health behavior of patients, and recommendations to promote a healthier community. Chapter Two covers development of the topic, explication of the problem, the clinical questions, and a discussion of the project design elements. Chapter Three provides an outline of the methodology that was used for the project. The chapter explored data collection and analysis, the project participants, and the measures taken to ensure that the DPI project complied fully with applicable ethical requirements. Chapter Four discussed the data analysis procedures, the results that the investigator obtained, the sample characteristics, and the observations regarding the impact of CRC screening. Chapter Five concluded the project with a comprehensive summary, summary of the findings, conclusions, recommendations for the project, and recommendations for future practice.
Literature Review
As is evident this far, the purpose of this quantitative quasi-experimental quality improvement project was to determine if or to what degree the implementation of AHRQ’s SATIS-PHI/CRC would impact colon cancer screening rates when compared to current practice among patients aged 50-75 in a primary care clinic in urban New York over four weeks. Already, there exists a tremendous amount of literature that explores various components of the project’s guiding clinical question. For example, investigators have already established that individuals in the 50-75 age group are among the populations that face the greatest risk of developing CRC (U.S. Preventive Services Task Force, 2021). Therefore, the purpose of this chapter was to shed light on the findings and insights from past research undertaken.
An organizational framework was adapted for this chapter. The chapter began with an outline of the theoretical foundations that underlie the project to set the stage. Next, the focus was given to the different theories and models that explained how using the SATIS-PHI/CRC toolkit to redesign the screening process can increase CRC screening rates. Next, the chapter explored some key themes and questions that have emerged from existing literature. Among the specific issues that this section addressed included the impact of the SATIS-PHI/CRC toolkit on CRC screening rates. The chapter concluded with a summary highlighting the most important results obtained from the literature review.
In conducting the literature review, caution was exercised to ensure a rigorous and extensive analysis was done. The PI conducted an in-depth examination of relevant literature. The critical measures adapted included providing that all the articles considered were published within the last five years, had been peer-reviewed, and addressed issues related directly to the clinical question for this project. Additionally, databases Pub Med, Ebscohost, CINAHL, and Google Scholar were used to find publications. These databases are appropriate because of their massive volume of collections and the valuable search tools that they incorporate to streamline finding literature.
Moreover, as part of the literature review, the principal investigator used keywords as colorectal cancer, colorectal cancer screening benefits, disparities, and colorectal cancer screening rates. These keywords yielded relevant results. Furthermore, only articles for which full texts were available were considered for inclusion in the review.
There is a gap in CRC screening. As numerous scholars have determined, despite CRC’s screening clear benefits, its uptake among patients has been low (Brenner & Chen, 2018). For example, Brenner and Chen (2018) noted that the administration of CRC screening falls short of expectation across many countries, given the proven advantages that screening offers. Huang and Huang (2017) also established that CRC screening rates are unacceptably minimal. If the U.S. is to make meaningful progress in its prevention of CRC, then it should be doing more aggressive screening promotions. The history of the slow adoption of CRC screening in the U.S. is long and complex. This history can be traced back to the early 2000s, when such organizations as the ACS began to issue guidelines to be used by practitioners in their screening procedures (Smith et al., 2019).
As Smith et al. (2019) reported, CRC is among the cancers that received early attention from the ACS, which issued regular updates and led an aggressive campaign to persuade patients to embrace screening. New and innovative solutions such as colonoscopy have been developed to improve CRC screening in the succeeding years. However, many Americans are still reluctant to undergo screening, resulting in an increased risk of developing the condition (Honein-AbouHaidar et al., 2016). Different stakeholders have historically disagreed on the best screening protocols, and tests for CRC are among the factors that experts have identified as responsible for the low screening rates in the U.S (Ransohoff & Sox, 2016).
Furthermore, the U.S. legal system is so convoluted and complex that practitioners and healthcare organizations have struggled to align screening procedures with applicable law, leading to a decline in screening rates (Ransohoff & Sox, 2016). Essentially, in addition to resistance from patients, the failure of CRC screening in the U.S. is also an outcome of regulatory and legal challenges that undermine the capacity of healthcare providers to perform screening. Throughout the literature and listed in this chapter are themes and subthemes identified as presenting barriers to CRC screening. The themes identified are CRC incidence and impacts, CRC risk factors and resource utilization, and colorectal cancer screening. The chapter provided a more detailed overview of the themes and subthemes identified.
Theoretical Foundations
Using the AHRQ’S SATIS-PHI/CRC toolkit to redesign the CRC screening process was the intervention that the current project examined to establish whether this solution can impact the CRC screening rate. The project was founded on the health belief model and the protection motivation theory. At their core, these theories underscore the need for comprehensive solutions to promote specific health behaviors and impact the design of disease prevention initiatives. The philosophy that will shape the project is the belief that screening allows for prevention and the early detection of illnesses. According to Lor et al. (2017), screening is generally effective because it provides healthcare practitioners early detection tools to fight diseases. Once a medical condition is in the early stages of development, appropriate measures can be instituted to keep the disease from further progressing, thereby preventing severe illnesses and, in some cases, death.
The successes that nations like the U.S. are witnessing in their war against different cancers result from timely age-appropriate screening (White et al., 2019; Yuan et al., 2018). It was hoped that when SATIS-PHI/CRC screening is rolled out at the New York hospital primary care clinic, it will generate diagnostic information that makes it possible for this facility to devise prevention and treatment solutions to increase screening and keep incidence rates of the condition at a low rate.
As previously mentioned, the project was founded on the health belief model and the protection motivation theory. Established by Ronald Rogers in the 1970s, the basic premise of protection motivation theory is that individuals tend to act in self-preserving ways. Essentially, Rogers believed that individuals would seek to eliminate risk or danger and restore their safety when confronted with a risk or danger (Jones et al., 2015; Rogers, 1975). Protection motivation theory identifies the severity of a threat, the probability of its occurrence, the mitigation measures available to an individual, and the individual’s capacity to activate these measures as among the key determinants of actions or behaviors that individuals institute to protect themselves (Westcott et al., 2017). Protection motivation theory presents several important implications with the present project:
- It suggested that driven by fear of developing CRC; patients will likely seek screening tests. Fear of getting the disease can promote protective behaviors and explain one’s cognition against threat and other coping behaviors.
- Since the participants in the project are aged between 50 and 75, the likelihood of developing CRC is significant. The high incidence of colorectal cancer can largely be prevented by adherence to routine preventive screening.
- This theory indicates that screening is a widely available and affordable solution that offers real protection against the condition. Therefore, the protection motivation theory can effectively identify successful health behavioral processes and interventions in encouraging, promoting, and getting patients to adhere to protective health behavioral practices. This argument makes the protection motivation theory valid and insightful in supporting and guiding the project.
Various researchers have used the protection motivation theory outlined above to understand populations’ health behaviors. For example, guided by this model, Khodaveisi et al. (2017) conducted a study to establish the effectiveness of a nutritional program designed for obese women. They determined that the program encouraged women to adopt healthier eating habits and concluded that the protection motivation theory accurately explains how individuals make decisions regarding their health. Basically, as Khodaveisi et al. (2017) determined, patients’ health choices are informed by the various factors that constitute the protection motivation theory. Furthermore, Aqtam and Darawwad (2018) confirmed that this theory is valuable for healthcare. After undertaking a literature review, they noted that the protection motivation theory also underlies the health promotion and disease prevention intervention that nurses design to influence research. Therefore, previous research has concluded that the model improves outcomes when incorporated into healthcare programs.
The health belief model is yet another theory whose elements are reflected in the project. Essentially, the health belief model posits that their belief determines an individual’s behavior toward a threat and their view on the effectiveness of available remedies. When individuals perceive the danger as significant and that the solution is effective, the health belief model suggests they are likely to implement it (Rakhshanderou et al., 2020). Moreover, the health belief model also recognizes that perceived barriers undermine the adaption of interventions. In contrast, self-efficacy provides individuals with the drive to undertake measures to limit their exposure to illness. This theory was devised in the 1950s by Godfrey Hochbaum, Irwin Rosenstock, and a group of scholars who sought to create a model that would account for the various factors that influence individual health behaviors (Rakhshanderou et al., 2020). In an insightful article that explored this theory and its implications for the design of healthcare programs, Rosenstock (1974) reiterated that individuals’ beliefs play a role in impacting their utilization of health resources.
The health belief model is important for the present project. First, the model underscores the need to ensure that patients believe that CRC is a real threat to their health and that screening is a simple, widely-available yet highly effective intervention that reduces the incidence and mortality of CRC over time. Second, the health belief model posits that when messages are delivered that target barriers to preventive care while focusing on self-advocacy and self-care self-efficacy, optimal successful behavioral change will occur. The health belief model and protection motivation theory are essential models of health behaviors and can influence or motivate an individual to care for oneself; for example, engaging in health promotional behaviors and preventing disease processes.
As with the protection motivation theory, the health belief model has also been validated in previous research. For example, Loke et al. (2015) used this model to examine the specific factors that influence pregnant women’s decisions regarding how to deliver. According to these scholars, the women’s decisions consider factors like perceived benefits, risks, and severity of the different delivery methods when making the decision. For example, during their study, Loke et al. (2015) noted that some women were worried about the risk of vaginal tearing that accompanies natural births and therefore opted for caesarian delivery. In addition, Siddiqui et al. (2016) also relied on the health belief model to investigate how households in Karachi, Pakistan incorporate healthy behaviors into their lifestyles. These scholars’ main observation was that self-efficacy and threat perception are among the key factors that underlie this population’s health decisions. Therefore, previous research has confirmed that the health belief model properly accounts for the behaviors and decisions that individuals and entire populations make.
The implementation of the project required supportive policies, systems, beliefs, and processes. For example, for the patients to agree to undergo screening, they must believe that screening is safe and effective and should be provided with services that streamline the screening process. After being screened, patients should get their results right away. Those diagnosed with CRC should then follow the steps for treatment and start right away. On the other hand, for patients free of CRC, appropriate education and support must be provided to protect them against the condition. With the treatment and preventive measures in place, the hope was for the New York hospital primary care clinic to report a significant increase in CRC screening rates.
Review of the Literature
Already, scholars have shed light on various aspects of CRC and the role of screening in preventing and lowering the number of patients who die prematurely from this condition. The following section examines some key issues that emerged from the literature and their relevance to the current project. The focus of this section is to demonstrate that CRC is already receiving considerable attention. However, as the discussion below reveals, some gaps can be addressed through additional research and scholarship.
CRC Incidence and Impacts
The incidence and effects of CRC are among the themes explored in the literature review. These issues are important because they help highlight the need for and the project’s significance. For example, as will be made clear below, CRC incidence has been on the rise in the U.S., and this condition is to be blamed for poor health and worse financial outcomes among patients. These negative impacts highlight the tremendous value that the project will add to healthcare.
Statistics
To understand the need for the current project, it is helpful to consider the scale of the CRC problem. CRC is well established as among the most pressing healthcare challenges confronting the U.S. today (Rawla et al., 2019). Existing research has warned about the alarming increase in the number of new CRC cases being diagnosed in the U.S. and other countries. Siegel et al. (2020) are among the scholars who have shed light on the incidence of this condition among Americans. Their text noted that in 2020, more than 147,000 new cases of CRC were reported in the U.S. In providing these epidemiological figures, Siegel et al. (2020) conducted a quantitative study.
They performed a rigorous analysis of data collected as part of a surveillance program developed by the National Cancer Institute. Siegel et al. (2020) also report that in 2020, the U.S. recorded at least 53,200 CRC-related deaths. Abualkhair et al. (2020) also confirmed that the incidence rates in the U.S. are worryingly high. Unlike Siegel et al. (2020), whose study focused on the general American population, Abualkhair et al. (2020) gave particular attention to those aged 40 and 49. As part of their cross-sectional quantitative study, the researchers reviewed surveillance data between 2000 and 2015 and determined that since adults in the 40-49 age groups are not prioritized for screening, many cases of CRC within this population go undiagnosed (Abualkhair et al., 2020). These findings undoubtedly indicate that CRC remains prevalent across different age groups in the U.S. and that urgent intervention is desperately needed.
Other regions have also received some attention from researchers who have sought to establish the global incidence rates of CRC. For example, Sierra and Forman (2016) joined forces to determine the burden of this condition on such Central and South American nations as Brazil, Argentina, and Uruguay. The quantitative study that Sierra and Forman (2016) conducted led them to find that despite the screening guidelines and protocols that these nations have in place, cases of CRC have been on an incline, thereby imposing strains on fragile healthcare systems that are already overburdened. To explain the increasing prevalence of CRC in South and Central America, Sierra and Forman (2016) noted that in this region, other health problems such as obesity, cigarette smoking, and poor nutrition could be responsible for the spike in CRC incidence.
This observation is important as it underscores the role that health behaviors play in influencing the prevalence of CRC. The present project sought to shed light on how such interpersonal issues as individual health behaviors affect exposure to CRC and the need to modify these behaviors to promote early CRC screening to reduce disease incidence. Other scholars have also conducted quantitative studies and confirmed that while the U.S. is among the nations with the highest rates of CRC, this condition is a global challenge that has spared no country (Arnold et al., 2017; Keum & Giovannucci, 2019). The worldwide spread of CRC means that the current project will yield insights that the project’s site, other U.S. facilities, and countries globally can adapt to harness the power of screening in their efforts to curb CRC.
The impacts of CRC have been devastating. Generating adverse health outcomes is among the effects that this condition is known to have. Reducing the quality of life of patients is one of the ways that CRC negatively affects human health. There appears to be a consensus among scholars and experts that even when patients recover fully from this condition, they struggle to restore their quality of life. For example, Ratjen et al. (2018) established the association between CRC and health-related quality of life (HRQOL).
Their study engaged 1294 individuals who had recovered from CRC and used the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 to determine how this condition had affected the participants’ lives. In addition to finding out that 175 of the patients enrolled in the study had died during the research, Ratjen et al. (2018) also noted that such issues as the decline in cognitive functioning were alarmingly common among the surviving participants. This observation led Ratjen et al. (2018) to conclude that CRC robs patients of their dignity, and this may lead to the cause of premature deaths in affected patients. This conclusion echoed the findings that Mrabti et al. (2016) obtained after investigating the health outcomes of CRC among a group of patients whose median age they calculated at 56 years. In this multicenter cohort qualitative study, Mrabti et al. (2016) were driven to determine the symptoms and quality of life issues among patients with CRC. They found that while such interventions as radiotherapy vastly improved symptoms, the patients experienced hardships that ranged from impaired physical and cognitive function to reduced role and social functioning (Mrabti et al., 2016). Essentially, CRC adversely impacted all areas of the lives of these patients
CRC Impacts
Imposing an economic burden on patients, families, and communities is another negative outcome of CRC. Numerous scholars have observed with great concern that individuals diagnosed with CRC incur huge costs as they seek treatment. Mariotto et al. (2020) have led the research community in warning about the economic impact of CRC treatment. The purpose of the quantitative study that they performed was to compute the average Medicare claims for CRC patients between 2007 and 2013 (Mariotto et al., 2020). They determined that initially, costs were $56,000 but can rise as high as $92,500 for patients requiring end-of-life care. Furthermore, Mariotto et al. (2020) determined that the treatment costs are highest among non-white and younger patients diagnosed with the advanced form of CRC. Those who had a history of cancer incurred even greater expenses. Blum-Barnett et al. (2019) joined Mariotto et al. (2020) in acknowledging that for many patients with CRC, the cost of treatment is prohibitively high.
For their qualitative semi-structured study, Blum-Barnett et al. (2019) held discussions with 14 individuals who had survived early-onset CRC. The main aim that these researchers sought to accomplish was to shed light on the impacts that CRC has had on the financial burden and health and the quality of life of these patients. They found that in addition to straining the patients’ finances, CRC had also resulted in emotional distress, harmed personal relationships, and reduced their quality of life. These adverse impacts highlighted the need for screening, a preventive measure that could hold the key to preventing the development of CRC and death. Others argued that all tests aren’t priced equally, and stool tests are just as effective as getting a colonoscopy to detect cancer when used according to recommended schedules. For example, although all screening methods are covered by insurance, in 2020, Medicare paid $16 for FIT and $509 for the Cologuard test (Jaklevic, 2021). A colonoscopy cost much more (Jaklevic, 2021). While all arguments on CRC impacts are reasonably stated, there needs to be some standardization about the consensus of CRC impacts and type of tests.
Prevention Interventions
Tremendous progress has been made in developing solutions for the prevention of CRC. Gonzalez et al. (2017) undertook an examination of some measures known to protect individuals and even entire communities against developing CRC. Their study took the form of a literature review and established that smoking cessation, nutritional counseling, and screening are among the interventions that significantly reduce CRC risk. Other researchers have confirmed that these prevention strategies are indeed effective. For example, following an extensive review of the outcomes and approaches to preventing CRC, Hull et al. (2020) identify smoking cessation programs as among the measures that have shielded populations against CRC and even improved recovery for those already diagnosed with the condition. Amitay et al. (2020) and Wilkins et al. (2018) also observed that when individuals quit smoking and adopt healthier lifestyles, their risk of CRC drops significantly.
The fact that there are numerous solutions known to offer protection raises questions about why the incidence of CRC within the American and global populations remains high. The current project presented screening as an approach that could persuade patients to take a keener interest in their health and become more involved in the efforts to contain CRC. Unlike the USPSTF, the Canadian Task Force on Preventive Health Care (CTFPHC) does not recommend using colonoscopy as a primary screening test for colorectal cancer due to lack of evidence of its importance. Instead, it advises for adults between 60 and 74 years of age, screening for colorectal cancer every two years using Fecal Occult Blood Test (FOBT), (either FOBT with a guaiac smear method (gFOBT), or Fecal Immunochemical Test (FIT) or every ten years using flexible sigmoidoscopy (Canadian Task Force on Preventive Health Care, 2016). For adults aged 50 to 59, the CTFPHC provided a weak recommendation for screening every two years using Fecal Occult Blood Test (FOBT), (either FOBT with a guaiac smear method (gFOBT), or Fecal Immunochemical Test (FIT) or every ten years using flexible sigmoidoscopy (Canadian Task Force on Preventive Health Care (CTFPHC), 2016).
While most CRC prevention strategies in use today are conventional, complementary and alternative solutions are rapidly emerging. Medicinal plants are among the remedies that are believed to protect against CRC. Aiello et al. (2019) assessed whether these plants offer real benefits and are indeed safe. These researchers focused attention on seeds, leaves, fruits, and plant roots often included in alternative therapies.
They established that in addition to being safe, the medicinal plants offer some relief and could effectively treat CRC. However, they also clarified that since the study was conducted in a laboratory, it was difficult to conclude that these benefits would be witnessed in a clinical trial. Huang et al. (2019) also conducted a mechanistic review to examine how the compounds found in medicinal plants function to prevent and treat CRC. They established that these plants can be beneficial and proposed that drugs for CRC treatment be comprised of elements derived from medicinal plants. However, as is the case with the study carried out by Aiello et al. (2019), this study has the shortcoming of being done in a lab. Further studies involving human participants are needed to establish whether plants and other forms of alternative and complementary medicine reduce exposure to CRC and help to extend the lives of those living with this condition.
While the risk of getting CRC may be high in some patient populations based on their family history, and there are many different modalities to testing, some individuals are reluctant to perform CRC-related testing due to fear. In addition, opponents argued that any abnormal results from tests other than a colonoscopy necessitate a colonoscopy to evaluate the abnormal test result. Again, this may lead to fear of testing and rejection, as most harms of screening for CRC are related to colonoscopy, including perforation (Doubeni, 2021).
CRC Risk Factors and Resource Utilization
In addition to the incidence and impacts of CRC, the literature review also highlighted the risk factors and the disparities in resource utilization among different populations. According to the literature, insufficient knowledge plays a vital role in any obstacles that limit access to care. Therefore, these subjects were explored to identify the most vulnerable groups most likely to benefit from the project.
Poverty
There is ample evidence that individuals from socioeconomically disadvantaged communities and backgrounds account for a disproportionate number of people living with CRC. Among the scholars who have warned that poverty poses a real danger to America’s campaign to reduce CRC incidence is Boscoe et al. (2014), who collaborated to investigate the relationship between the prevalence of CRC and poverty. Relying primarily on diagnostic data collected between 2005 and 2009 in Los Angeles, Boscoe et al. (2014) confirmed that being poor means that one is significantly more likely to become ill with CRC. In a study conducted in conjunction with the CDC, Kollman and Sobotka (2018) also set out to shed light on whether and how poverty contributes to the incidence and adverse outcomes of CRC.
Their study compared CRC rates in poor and affluent areas in Ohio. Through quantitative research, Kollman and Sobotka (2018) reached the same conclusion that Boscoe et al. (2014) arrived. They found that the incidence rate of all cancers, including CRC, was at least 19% higher among the poorer counties than in the wealthier neighborhoods. Many other researchers have made similar observations and called for the development of solutions targeted at poor communities (O’Connor et al., 2018; Tanaka et al., 2020). The question of poverty is relevant to the present project because it evaluates a low-cost intervention and can be modified to suit the unique needs and the barriers that poor patients encounter as they seek to utilize healthcare resources.
The impact of poverty on CRC rates can be seen when one compares the situation in different countries. As Arnold et al. (2017) found out, an alarming increase in CRC incidence has been observed among low and middle-income countries in the recent past. On the other hand, in industrialized nations, the prevalence of CRC is dropping or stabilizing. Schleimann et al. (2020) concurred with Arnold et al. (2017) that today, poor communities are bearing the brunt of CRC. In addition to finding that low and middle-income nations accounted for an increased proportion of CRC cases, Schleimann et al. (2020) also reported that in poorer countries, survival rates for those who develop CRC are depressingly slim due to fragile and inadequately-funded healthcare systems. Abotaleb (2018) also found that communities in developing countries are more susceptible to developing this cancer due to a lack of investment in CRC prevention measures.
Furthermore, according to Abotaleb (2018), in such developing countries as Egypt, the cost of CRC treatment is unaffordable for many, and it is therefore not surprising that underdeveloped nations lead the world in CRC prevalence. Moreover, the impact of poverty is so significant that, as Wise (2016) noted, children who grow up in poor neighborhoods are more likely to develop CRC. Thus, it is clear that for efforts to curb CRC to yield positive results, attention must be shifted toward addressing the socioeconomic determinants of health.
In addition to identifying poverty as among the risk factors for CRC, existing research has outlined the specific mechanisms through which poverty fuels CRC incidence and mortality. Among the scholars who have established the link between poverty and CRC risk are; Syriopoulou et al. (2019), who undertook a population-based study involving 470,000 participants from diverse socioeconomic backgrounds and diagnosed with CRC. According to Syriopoulou et al. (2019), poorer patients face the worst prospects of recovery and the most significant risk of developing CRC because of under-investment in their communities. Henry et al. (2014) note that the direction of the association varied globally and argued that while it has been reported that CRC and subsite-specific incidence rates vary by socioeconomic status (SES), in the USA and Canada, lower SES has been associated with a higher risk of CRC. In contrast, in Europe, Australia, and South Korea, lower SES has been associated with a lower risk of CRC (Henry et al., 2014).
The authors further commented that, while SES is not a direct determinant of the incidence differences by subset, variance in incidence rates among SES groups is likely due to common CRC risk factors, which vary by SES. Such factors as physical inactivity, unhealthy diet, smoking, obesity, and poor access to and underuse of screening services for early detection and removal of precancerous polyps are determinants noted (Henry et al., 2014). Essentially, these scholars blame governments for failing to prioritize the well-being of poor populations. The fact that poverty reduces access to such resources like parks and gyms, as well as good nutrition that is needed to prevent and treat CRC, also helps to explain why the poor account for the majority of CRC cases and deaths (Jackson et al., 2016; Rock et al., 2020).
The project accounts for poverty’s role in CRC incidence. Coughlin (2020) carried out a systematic review and concluded, to address social determinants of colorectal cancer, effective interventions are needed that account for the social contexts in which patients live. The review also explains that colorectal cancer incidence rates are positively associated with income and other measures of socioeconomic status. In contrast, low socioeconomic status tends to be associated with poorer survival (Coughlin, 2020). While it does not mainly target poor communities, this project recognizes the hardships these groups face and steps needed to ensure that no participant encounters any unnecessary obstacles while attempting to undergo screening for CRC. Since the start of the project, the project’s site has begun working with its patients to find ways to improve ease in scheduling and getting them to the facility on their test date.
Role of Geography
Geography is another risk factor for CRC. Generally, individuals in rural areas face a higher risk of becoming sick with CRC when compared to their counterparts in more developed urban centers. This is one of the main observations that Rogers et al. (2020) made after undertaking a population study involving 4,660 men drawn from various areas in Utah. In addition to finding that the patients from rural Utah were more likely to have CRC, Rogers et al. (2020) also established that these patients had lower survival rates. This finding is essentially in line with the study outcomes that Carmichael et al. (2020) performed. The purpose of this quantitative quasi-experimental quality improvement project was to determine if or to what degree the implementation of AHRQ’s SATIS-PHI/CRC toolkit would impact colon cancer screening rates when compared to current practice among adults aged 50-75 in a primary care clinic in urban New York, over four weeks. One of the results that Carmichael et al. (2020) obtained was that the prevalence of CRC in rural areas was higher than in urban regions.
However, they also found that when they undergo screening, patients in the rural regions significantly reduce their CRC risk, thereby helping to eliminate the regional disparities. Other researchers have echoed this finding and have endorsed screening as a safe, cost-effective, and highly impactful approach to lowering inequalities and improving health (Alyabsi et al., 2020; Swaminathan et al., 2020). The hope was that the present project would add to the existing body of knowledge that explored the solutions that can be implemented to ensure that communities in rural areas enjoy the same level of access to preventative resources and treatment options available to their counterparts in urban centers.
This project aimed to achieve this by ensuring that the participants involved in the project are from different ethnic groups and reflect the demographic and regional diversity of the U.S. Scholars have attempted to shed light on the mechanisms that underlie the regional disparities in CRC prevalence and mortality. Among the factors that have been blamed for the differences between rural and urban areas is the under-investment in rural regions. For example, Alyabsi et al. (2020) performed a retrospective cohort study to establish the amount of time patients from rural and urban areas spend traveling to their practitioners to undergo CRC screening. They noted that, on average, those from rural regions were forced to travel long distances as their immediate communities lacked screening programs. To explain this finding, Alyabsi et al. (2020) suggest that the U.S. has failed to invest in healthcare infrastructure designed to serve rural populations adequately. Zahnd et al. (2021) also explained why rural areas continue to lag behind their urban counterparts.
According to Zahnd et al. (2021), it is crucial to consider race and ethnicity’s role in America’s healthcare policy to understand the disparities fully. Zahnd et al. (2021) conducted an extensive data analysis to determine how age mediates CRC incidence in rural and urban regions. They found that while rural areas generally fall behind in CRC incidence, rural regions with the majority of Native American populations had some of the highest CRC prevalence rates that they recorded. This result is significant as it indicates that if the U.S. is to make any meaningful gains in increasing CRC screening and reducing CRC incidence, it must pursue racial justice and strive to eliminate disparities. In a 2017 article on a discussion on rural communities and colorectal cancer screening, Dr. Djenaba Joseph, medical director of CDC’s Colorectal Cancer Control Program, admitted that screening rates are lower in rural areas, where geography causes barriers like lack of access to providers and lack of specialists or access to those specialists (Miller, 2017). However, she suggested that when providers engage their age-appropriate patients and talk to them at every opportunity about colon cancer screening, rates of screening increase (Miller, 2017).
According to the article, Joseph further emphasized that knowledge about available resources helps providers and patients decide on the best CRC screening test. Dr. Joseph also pointed out that evidence-based interventions such as reminders work well in rural areas and can help small communities improve screening rates. For patients, who often deal with acute health problems or multiple chronic problems, she suggested reminders as valuable tools for patients and chart reminders prompt for providers (Miller, 2017). The current project is among the resources that the nation can leverage to establish a new dispensation where all Americans enjoy unhampered access to such services as screening. Being established on such theory as the health belief model, the project demonstrated that when they encounter barriers like limited access to screening services, patients are less likely to sign up for CRC screening without education.
Health literacy
Health literacy is yet another factor that is known to influence CRC outcomes. Essentially, health literacy is concerned with individuals’ capacity to locate, dissect and apply health information. For best health outcomes, patient populations must be health literate (McDonald & Shenkman, 2018; Singh & Aiken, 2017). Health literacy has emerged as a resource that plays a crucial role in protecting populations against colorectal cancer and improving the survival of patients who already have the condition. Arnold et al. (2019) performed a randomized controlled trial that concluded that health literacy is an essential component of programs for curbing CRC. Working with a group of patients aged between 50 and 74 from four rural communities in the US, Arnold et al. (2019) sought to establish whether such techniques for enhancing health literacy as the use of pamphlets containing information on CRC and the teach-back approach have any impact on CRC screening.
As one would expect, Arnold et al. (2019) found that these strategies were highly effective as they encouraged the participants to seek screening and other CRC-related health services. Conversely, low levels of health literacy have been shown to increase the risk of developing CRC. For instance, Woudstra et al. (2019) found that patients who lack adequate health literacy skills are often unable to take advantage of the various programs and services developed to protect them against CRC. Furthermore, without these competencies, patients are usually unaware of these services, and when they understand that the programs exist, they lack the knowledge regarding how to access them.
Horshauge et al. (2020), Mantwill et al. (2015), and Stormacq et al. (2019) pointed out that the concept of health literacy is of particular interest as health literacy has been suggested as a potentially modifiable factor by which health disparities, such as inequalities in CRC screening, can potentially be reduced (Horshauge et al., 2020; Mantwill et al., 2015; Stormacq et al., 2019). However, others added that literature regarding the association between health literacy and CRC screening uptake is inconsistent. Some studies concluded that inadequate/limited health literacy is associated with lower screening uptake (Horshauge et al., 2020; Solmi et al., 2015), whereas other studies find no association (Miller et al., 2007; Wangmar et al., 2018). The current project recognized the importance of health literacy. Among the key components of the project was patient education. As part of the education packet for the project, all participants were provided with information on the benefits of screening. Furthermore, by highlighting the value of patient education, this project provided healthcare providers and policymakers with the evidence needed to incorporate health literacy programs into their efforts to tackle CRC.
Colorectal Cancer Screening
The third theme that emerged from the literature concerns CRC screening. During the literature review, it became evident that scholars agreed that when administered early, screening can indeed insulate populations against CRC and vastly improve survival outcomes when diagnosed. Therefore, examining the role of screening is deemed important to establish the value of the present project.
Current State of Screening
The current state of CRC screening is one of the numerous sub-themes upon which scholars have paid particular attention. Evidence suggests that many patients who are eligible for CRC screening have not undergone this procedure. For example, in 2016, only 68.8% of adults in the 50-75-year age group had been screened for CRC, according to a CDC report (CDC, 2020). This figure represents a 1.4% increase from the previous year. However, even as the U.S. celebrates the progress that it is making in persuading its citizens to undergo screening, it is recognized that millions remain susceptible to CRC because they are yet to be screened.
Various factors are behind the gains that the U.S. has made in increasing the acceptance and use of screening services among its patients. In their report on screening rates in the country, Montminy et al. (2019) commended the U.S. for the valiant and unrelenting commitment that it has demonstrated. These scholars note that enabling legislation that has facilitated access to screening programs and the development of non-invasive screening protocols are interventions that have fueled the increase in the number of Americans agreeing to this procedure. The measures that the U.S. has instituted demonstrate that it is dedicated to fighting CRC.
As the discussion above has shown, CRC screening rates in the U.S. are on the rise. However, it should be noted that there are certain areas where the U.S. is performing poorly. For example, the nation has been unable to ensure that all its citizens enjoy access to screening services. As White and Itzkowitz (2020) observed, racial minorities have some of the lowest screening rates in the US. A previous section pointed out that in many minority neighborhoods, healthcare services are in short supply. Therefore, it is not surprising that minorities in the U.S. are less likely to take advantage of screening programs.
Moreover, historical injustices that some of these communities have endured have harmed their confidence and trust in the medical community. For example, according to Taylor (2019), the slow uptake of medical programs among African Americans can partly be blamed on racism and inequality. Unless the U.S. assures this population that screening is safe and helps save lives, African Americans and other minorities will continue to reject this procedure. The present project addresses the concerns these groups have raised by ensuring that all communities are fairly represented, and that the insights generated by the project are used to improve access to screening services across the country.
Screening Challenges
As it attempts to make CRC screening available to all, the U.S. has encountered several challenges. DeGroff et al. (2018) highlighted some hardships that have frustrated efforts to screen the most vulnerable populations. In their discussion on measures that the nation can institute to improve screening rates, DeGroff et al. (2018) expressly identified low educational attainment, poverty, and racial disparities as issues that are undermining screening programs. In particular, according to DeGroff et al. (2018), communities across the U.S. cannot appreciate the value of screening without adequate education.
Furthermore, these scholars contend that poverty limits access to screening programs and racial disparities. This means that minorities encounter the harshest difficulties when they attempt to undergo screening. Even individuals with health insurance can run into problems resulting in their avoidance of the test. Bone et al. (2020) agreed and expressed that individuals with insurance paying a high detectable cost may go without doing their screening tests. This they attribute to the delay in insurer payouts resulting in the patient having to pay the total price of their test until their deductible is met. This could result in complete avoidance of the test by patients. These challenges demonstrate that the U.S. needs to implement systemic reforms to eliminate such issues as poverty and racism that have made it difficult for healthcare providers to deliver screening interventions to all communities. The current COVID-19 pandemic is another issue that has been implicated in the low levels of CRC screening.
Cancino et al. (2020) found that this pandemic has forced the U.S. to divert its resources and attention from such healthcare challenges as cancer toward the coronavirus. As a result, today, patients encounter obstacles when trying to receive screening for cancers like CRC. It is highly likely that due to COVID-19, thousands of CRC patients will die, and new cases that could have been prevented or detected, and treated early through screening, will progress and cause serious complications (Patel et al., 2021). While the current project does not particularly address how to resolve the challenges that adversely affect screening, it will demonstrate that through such strategies as evidenced-based personalized interventions and inclusive solutions, it is possible to improve screening rates among diverse patient populations.
Benefits of Screening
The benefits of CRC screening cannot be overstated. One of the advantages of this procedure is that it helps to reduce disease incidence. According to Khalili et al. (2020), in addition to being highly cost-effective, CRC screening facilitates the detection of CRC cases. Among communities with robust screening protocols, CRC rates are lower because these populations receive insights into the strategies and measures they can adopt to reduce their risk of CRC further. Levin et al. (2018) also found that screening pushes down the rates of CRC incidence. Unlike Khalili et al. (2020), who undertook a systematic review, Levin et al. (2018) performed a large community-based study to establish how screening would impact CRC incidence. They confirmed that, the screening program had resulted in significant improvements in screening participation and a drop in CRC incidence within a short period. This finding gave the PI the confidence that the project would demonstrate that a redesign of the screening process using the SATIS-PHI/CRC would be an effective toolkit for increasing screening rates once implemented.
In addition to reducing CRC incidence, screening has also been shown to enhance patient survival. Bone et al. (2020) are scholars who have linked screening to improved CRC survival. After holding a round table evaluation, Bone et al. (2020) found that their quality of life had been boosted and their lifespan extended, thanks to screening. Brenner et al. (2016) obtained a similar result after investigating CRC progression among patients who had received screening. According to Brenner et al. (2016), screening created a favorable prognosis for these patients. This finding is essential as it informed the hypothesis that guided the project. It is hoped that the project will show that evidence-based personalized screening dramatically lowers the risk of death.
Another benefit of screening is that it is a cost-effective solution. According to Khalili et al. (2020), the implementation of screening programs is not overly costly. Bone et al. (2020) and Subramanian et al. (2017) also found that among the qualities that make screening appealing is its low cost. More importantly, screening helps to reduce the cost of care and the huge healthcare burden that patients would otherwise incur if they were to be diagnosed with late-stage CRC. Additionally, screening bolsters the economy by preventing the productivity losses caused by ill health and death due to CRC. AHRQ’s System Approach to Tracking and Increasing Screening for Public Health Improvement of Colorectal Cancer toolkit was created to inform and educate new users and increase CRC screening programs and techniques. The toolkit is intended to assist primary care practices in providing guideline-based preventive health care to age-appropriate patients, who have a moderate risk for CRC, and who are not updated with their screening (AHRQ, 2018b).
The toolkit provides education for patients and providers about CRC screening and assists practices in encouraging, facilitating, and providing screening (AHRQ, 2018b). The AHRQ’s System Approach to Tracking and Increasing Screening for Public Health Improvement of Colorectal Cancer toolkit includes stool test, colonoscopy, flexible sigmoidoscopy, or barium enema x-ray. AHRQ maintained that the toolkit was developed consistent with research findings of effective CRC screening programs and techniques. Therefore, this toolkit includes tools, process guidelines, tips (based on lessons learned), and evidence of the intervention’s effectiveness (AHRQ, 2018b). Persons who undergo CRC screening using multiple modalities were counted only once (AHRQ, 2018b). This included only patients accessible in the primary care clinic’s electronic medical record systems, where patients’ electronic health records can be reviewed.
The tool is ideal for the primary care clinic as it has seen a decline in colon cancer screening over the last couple of years. The pandemic has contributed to an even further reduction in all screening in the clinic, making preventive care an essential priority for all stakeholders. Screening rates now average a meager 38% over the previous three years at the clinic. Before the pandemic, the rate of CRC screening was at 55%, which was well below the national average, but dramatically plummeted during the heart of New York’s COVID-19 crisis. The AHRQ toolkit is based on the premise that busy primary care practices could benefit from assistance in carrying out population-based screening programs (AHRQ, 2018a). According to Zapka, a central entity provides this assistance by identifying eligible patients for but not up to date in their CRC screening.
It then sends invitations for patients to be screened, provides educational screening information and material to those patients, tracks whether patients get screened, and sends reminders to those who did not screen. Reminders are also issued to the practices to follow up with screened patients (Zapka, 2008). This toolkit is ideal for the practice to attain its desired outcomes of increasing the CRC screening rate while reducing its incidence. The toolkit prides itself on being patient-centered emphasizing on education and quality patient’s outcomes.
More importantly, there is evidence that the tool is helpful for screening. For example, Sarfaty et al. (2012) evaluated how various screening tools; including the SATIS-PHI/CRC toolkit facilitate the screening process. While they agreed that this toolkit has some flaws, such as the difficulty of contacting non-responding patients, it was found to be generally helpful and reliable. Additionally, AHRQ (2018a) also established that, the SATIS-PHI/CRC toolkit generates significant and sustained increases in screening rates compared to other available solutions. In light of this evidence, the PI concluded that the SATIS-PHI/CRC was appropriate for the project.
Summary
The literature review was extensive and has shed light on various issues that affected the project. The review has highlighted the burdens that CRC imposes on patients and their communities, the risk factors that expose individuals to this condition, and the role that screening plays in offering protection. These themes echoed the essence of the project. The project aimed to demonstrate that through a redesign of the CRC screening process using AHRQ’s System Approach to Tracking and Increasing Screening for Public Health Improvement of Colorectal Cancer toolkit, the U.S. can make more significant progress in protecting the most vulnerable populations and limiting the harm that CRC causes. Furthermore, the literature has shed light on some gaps that the project sought to fill. For example, most existing literature has focused on general screening strategies, with evidenced-based personalized screening receiving little attention. The project addressed this gap by investigating the impact of redesigned screening approaches using the SATISPHI/CRC toolkit.
The health belief model and the protection motivation theory are the models that guided the project. These models are valid and reliable tools for assessing how different programs influence health behaviors. The project incorporated these models and demonstrated that supportive services are effective in helping patients understand that they possess the necessary self-efficacy tools to inform and encourage them to screen so they can be shielded from CRC. During the literature review, it was noted that most studies have been quantitative, and have involved actual human participants. This finding validated the data collection techniques and instruments, the design and the project’s methodology.
The project builds on previous research that aimed to determine whether redesigning the screening process using the SATIS-PHI/CRC toolkit would effectively increase screening rates among patients between 50 and 75 years at a primary care clinic of a New York hospital. This project aimed to advance the topics and the theories covered in the literature review by exploring the different factors that shape the effectiveness of AHRQ’s evidenced-based toolkit and highlighting the importance of systemic reforms that make screening available to all patients.
Chapter Two has shed light on various important issues related to the project. It has discussed the role of screening and the dangers that CRC poses to human health. Furthermore, this chapter has also addressed the theories that will guide the project. In Chapter Three, an outline of the methodology used for the project is provided. Key topics that the third chapter explored include data collection and analysis, the project participants, and the measures taken to ensure that the project complied fully with applicable ethical requirements.
Methodology
Colorectal cancer is among the conditions that impose heavy burdens on the American healthcare system. According to Eaglehouse et al. (2019), patients who develop CRC can expect to incur as much as $159,729 when they seek care in the U.S. In addition to the high cost, CRC is also responsible for thousands of deaths every year. For example, in 2021, projections show that CRC will result in more than 52,980 deaths (American Cancer Society, 2021b). The high mortality and healthcare expenditure underscore the need for urgent interventions.
It is well established that screening is one of the tools that play a crucial role in managing such conditions as CRC. However, it has been noted with great concern that many individuals, particularly; those most at risk for developing CRC, do not undergo screening in the U.S. For example, according to the Centers for Disease Control and Prevention (CDC), out of every three adults in the 50–75-year age group, only one has been screened for CRC (CDC, 2013). The low screening rates help explain why CRC is prevalent in the U.S. The current project sought to address this problem by investigating whether the redesign of screening using the SATIS-PHI/CRC toolkit improves screening rates.
The purpose of this quantitative quasi-experimental direct practice improvement project was to determine if or to what degree the implementation of AHRQ’s SATIS-PHI/CRC toolkit would impact colon cancer screening rates when compared to current practice in adult patients aged between 50-75 years, in an urban New York hospital primary care clinic, over four weeks. This project was inspired by the need to devise impactful yet straightforward solutions that can help to reduce the burden that CRC imposes. Furthermore, the project seeks to protect the dignity and lives of adults aged 50 to 75 years who make up one of the most at-risk populations.
Statement of the Problem
This American Cancer Society (ACS) (2021a) emphasized that colorectal cancer (CRC) is the third most common cancer and the second leading cause of cancer death in the United States. According to ACS (2021a), routine screening and resultant early detection through a range of acceptable strategies (colonoscopy, fecal testing, among others) are helpful and cost-effective in reducing CRC incidence and mortality. However, while there is an abundance of evidence that suggests that early screening saves lives, it is not known if, or to what degree, the implementation of AHRQ’s SATIS-PHI/CRC toolkit would impact colon cancer screening rates, when compared to current practice among patients 50-75 in a primary care clinic, in urban New York, over four weeks.
Clinical Question
The primary clinical question that guided the project reads: to what degree the implementation of AHRQ’s System Approach to Tracking and Increasing Screening for Public Health Improvement of Colorectal Cancer toolkit would impact colon cancer screening rates when compared to current practice in patients 50-75 in an urban New York primary care clinic over four weeks? The project’s aimed to establish whether a redesign of the CRC screening process using the SATIS-PHI/CRC toolkit would increase CRC screening rate at an urban New York primary care clinic. On the one hand, the SATIS-PHI/CRC toolkit is the independent variable. On the other hand, the screening rate is the dependent variable. The project sought to establish the nature of the association between the two variables: the SATIS-PHI/CRC toolkit and an increase in CRC screening rate.
The principal investigator applied the quasi-experimental approach to answer the clinical question outlined above. A quasi-experimental design is inexpensive, can be generalized, and has better external validity than random control trials (Schweizer et al., 2016). The Healthcare Effectiveness Data and Information Set (HEDIS measure) Colorectal cancer screening tool was the primary instrument that the investigator deployed for data collection. Essentially, this toolkit made it possible to track the progress of patients who undergo CRC screening at the primary care clinic. Healthcare Effectiveness Data and Information Set (HEDIS), is a tool used by more than 90 percent of America’s health plans to measure performance related to care and service (NCQA, 2017). The HEDIS measure pertaining to colorectal cancer screening focuses on patients ages 50-75 who receive the appropriate screening for colorectal cancer (National Committee for Quality Assurance (NCQA), 2017). Multiple test types meet the requirements for screening completion under the HEDIS measure.
Tests include screening colonoscopy every ten years (preferred recommended option) or screening flexible sigmoidoscopy every five years, computed tomography (CT) colonography every five years, screening fecal occult blood test (FOBT) annually, and FIT DNA (i.e., Cologuard) test every three years, just to name a few (National Committee for Quality Assurance (NCQA), 2017). Data was gathered from the patients’ electronic health record (EHR) in the primary care clinic’s EPIC electronic medical record (EMR) database. The urban primary care clinic hosting the inquiry provided information on the screening rates among patients in the period immediately following the conclusion of the project to determine the impact of SATIS-PHI/CRC on the dependent variable. On the other hand, screening is concerned with increased preventative care and focuses on health promotion.
The quasi-experimental method selected for the project is appropriate for various reasons. First, this approach is routinely used to establish causal relationships among variables (Kim & Steiner, 2016). Therefore, it is expected that this design will shed light on whether the redesign of the CRC screening process led to an increase in the rate of screening. Secondly, the quasi-experimental approach has been found to enable investigators to obtain accurate, reliable, and valid conclusions (Maciejewski, 2020). Therefore, the present project’s findings can be accepted as credible and be incorporated into the management of CRC.
Project Methodology
The current project utilized the quantitative methodology. The project involved participants being screened for CRC using a redesigned approach. Data were collected pre and post-intervention, then compared to determine changes in screening rates. At the start of the project, the CRC screening status of all of the 100 participants was determined. It was established that only three patients had been screened for CRC prior to the project. This process made it possible to measure the impact of the AHRQ’s SATIS-PHI/CRC tool kit on CRC screening at the end of the project by comparing the number of new CRC cases detected through the screening to the cases recorded at the beginning of the project. Data on CRC screening rate was gathered 30 days following the adaption of the project.
It should be noted that after depersonalization, before the screening was administered, for each of the 100 participants, the CRC status was established by examining data held in the site’s records. Comparing the pre and post-intervention data analysis was essential to collect new insights on CRC screening rates using the SATIS-PHI/CRC toolkit. On the other hand, the HEDIS measure was deployed to assess the overall effectiveness and impact of the intervention (screening using the SATIS-PHI/CRC toolkit). The PI performed a comparative analysis of the pre and post-implementation screening data to determine if there was a change in the screening rate.
The quantitative methodology was appropriate primarily because it allows for the recruitment of a larger sample than a qualitative method, making the data collection process more accurate and objectively minimizing the risk of bias (Daniel, 2016). Moreover, the quantitative approach was selected because it allows for a faster and more accessible collection of data, is more generalizable, and helps to guarantee the reliability and validity of the conclusions reached. Additionally, the quantitative method allows for other researchers to replicate the project. Finally, quantitative methodology fits the project because the outcomes are measurable, and it allows for the measurement of outcomes for comparison. Given these advantages, the quantitative method is ideal as it was expected to generate reliable, valid, and credible insights, and it can be generalizable and can be used to improve patients’ outcomes and healthcare practices.
Project Design
The project used a quasi-experimental design. This design was selected because it delivers the advantages of experimental approaches while eliminating the shortcomings that undermine the reliability of experiments. For example, one of the main strengths of the quasi-experimental design is that it offers the opportunity to determine if there exists a causal connection among the variables that an individual is attempting to investigate (Kim & Steiner, 2016). Another advantage of the quasi-experimental approach is avoiding the challenges such as the complexities and the high costs associated with fully experimental methodologies. Most importantly, the quasi-experimental strategy is ideal because it aligns with the current project’s goal. For example, the main objective of the project was to increase CRC screening rates. The quasi-experimental approach facilitates this goal achievement by allowing for the comparison of screening rates pre and post-intervention. Moreover, the quasi-experimental design is the best fit for the project because it is nonrandomized (Thomas, 2020).
Population and Sample Selection
The sample included in the project was drawn from adults aged between 50 and 75. This population was chosen because it comprises individuals most at risk for developing CRC. In its report on the prevalence and risk factors for CRC, the CDC (2021b) clarified that adults in the 50–75-year age bracket are particularly vulnerable and that screening programs should be targeted at this group. Since the project sample reflects the profile of the larger population, it is expected that the insights from this project will be such that they can be applied to the 50–75-year-old population and that the project’s conclusions can be generalized. While the project population is somewhat limited, the project will still present implications for the total population, which is made up of teenagers and adults in the U.S. Literature show that while those aged between 50 and 75 faces the most significant risk of CRC, this condition has also been diagnosed among young adults and even teenagers (Siegel et al., 2020).
Age was the primary factor that was used to recruit participants. The larger population that the sample represents was the group of 50–75-year-old Americans who were most at risk for developing CRC. Data from ACS (2020) statistics showed that in early 2019 there were more than one and a half people alive with colorectal cancer, with older individuals accounting for the bulk of this number (American Cancer Society, 2020). Therefore, the project sample comprised n=100, 50–75-year-old adults receiving care at a New York hospital urban primary care clinic. Convenience, costs, and practicality are the main factors that were considered when setting the sample size at n=100. However, practical considerations are the main issues that informed the sample size.
Basically, in consultation with the authorities at the site, the PI determined that a sample of 100 was large enough to collect credible and high-quality insights while still ensuring that the project could be practically conducted. While convenience, practicality and cost are the primary considerations that informed the sample size, previous research was also consulted when determining the number of patients to include in the project. For instance, according to Brysbaert (2019), whereas a sample should be as large as possible, a sample size of 100 is acceptable as it meets basic power requirements. More importantly, as discussed in detail in a later section, a power analysis was conducted to establish the appropriate sample size for the project. The goal of the power analysis was to determine the appropriate size of the sample to ensure that the project’s findings could be generalized to the larger population.
No restrictions were imposed on the participants based on such factors as their race, gender, or socioeconomic background. However, the participants were expected to meet the age requirement to participate in the project. This was done to minimize bias and ensure that the sample represented the larger 50-75-year-old population. While it remains true that the project did not impose any undue requirements or restrictions on the participants, they were expected to meet the criteria for inclusion in the project. First, all the participants needed to be between 50 and 75. However, they were invited to participate in the project provided that they received care at the site and met the age requirement. Ideally, the sample size should be large enough to reflect the dynamics and experiences of the target population properly.
However, for the present inquiry, it proved challenging to achieve this ideal based on power analysis. Having adequate resources to solve statistical problems is often involved in selecting the sample size of a project. Usually, when estimating the sample size, one must consider such issues as the size of the population and the degree of accuracy that they wish to achieve (Binu et al., 2014). Assuming that the project satisfies the minimum requirement of a power value of 80%, the PI would be required to recruit more than 800 participants, far much higher than the 100 participants recruited for the project.
However, following lengthy discussions with the site’s authorities, it emerged that a project with 800 participants would be unfeasible for various reasons. First, the hospital made it clear that the total number of patients aged 50-75 who were receiving treatment at the time was only about 256. This fact implied that the ideal sample size could not be achieved. Secondly, the hospital faced severe financial and other constraints that undermined routine care delivery when the project was conducted. Therefore, requiring the hospital to screen 800 patients would have stretched its already thin resources even further. Thirdly, as noted earlier, the project was conducted over four weeks. Given the time limitation, it was not possible to screen 800 individuals. These factors were considered critically, and after further consultations with the hospital’s leadership, it was determined that the hospital could screen 100 individuals over the four weeks without compromising other operations.
To recruit the 100 participants, the PI used the quota sampling method. One of the key advantages of this sampling approach is that it does not require randomization, meaning that researchers can rely on their judgment to determine the sample size (Taherdoost, 2020). However, the main drawback of this technique is that it is of a non-probability variety. As such, questions can be raised about the reliability and validity of the project. Essentially, the investigator issued an open invitation to all patients receiving care at the urban clinic of the New York hospital. However, the PI also required interested individuals to meet set criteria.
For example, all participants had to be in the 50-75 age brackets and must be current urban primary care clinic patients for the inclusion criteria. Individuals outside of the age range and did not receive treatment at the clinic were excluded from the project. The interested patients were sent an anonymous survey questionnaire to establish if they satisfied the requirements. While the questionnaire did not require the prospective participants to provide their names, they were still required to indicate their age and such other demographic data as ethnic background. To ensure that only eligible patients were involved in the project, they were required to return the questionnaire when they visited the hospital for screening. In addition, the practitioners involved in the project used alphanumeric code names to track the patients, which helped safeguard the participants’ privacy while ensuring that the project was correctly performed.
As part of the project, the PI obtained site authorization. To be granted permission to undertake the project, the PI engaged the site’s leadership on the value of the project and the measures that would be instituted to guarantee the rights of the participants. For example, although the project posed less than minimal risk to the participants, all collected data and personal information were preserved under HIPAA standards and de-identified. Any detail, including patient information, location, and caregiver, was protected per the site’s policy. In addition, an informed consent document or recruitment information was not required for recruitment at the site because the PI was involved in a practice improvement project and not research. However, a consent waiver was obtained from the university’s IRB board.
With the help of one nurse, flyers and the AHRQ’s SATIS-PHI/CRC toolkit survey were mailed out to the participants. As noted above, the patients who eventually took part in the project were selected using quota sampling. With the help of practitioners at the clinic, the surveys were then mailed to the patients. The flyers provided education on CRC screening, and the survey provided questions to determine who met the inclusion criteria. Among other steps to secure participant confidentiality was limiting access to the investigative data through passwords and encryption. The project was confined to the urban primary care clinic to minimize costs and ensure that the project did not cause any interruptions to the site’s operations. The project’s sample was taken from the group of eligible patients. All participants met the inclusion requirements of the project. As noted previously, quota sampling was used to recruit the participants. Essentially, all interested and eligible participants were invited to participate in the project. When the target of 100 was reached, no other admissions were permitted, and the sampling process was terminated.
Instrumentation
The PI used a validated instrument to gather data instead of relying on existing data whose reliability and accuracy could not be guaranteed. The instrument used for data collection was the NCQA HEDIS measure. Using this tool, the PI tracked the patients who had been screened for CRC. Developed in 1991, the NCQA Healthcare Effectiveness Data and Information Set (HEDIS) is a comprehensive set of standardized performance measures designed to provide purchasers and consumers with the information they need for reliable comparison of health plan performance (Centers for Medicare & Medicaid Services, 2021).
HEDIS measures relate to significant public health issues like colorectal cancer, heart disease, smoking, asthma, and diabetes. HEDIS measures performance data to identify opportunities for improvement, monitor the success of quality improvement initiatives, track progress, and provide a set of measurement standards that allow comparison with other plans. In addition, data allow the identification of performance gaps and the establishment of realistic targets for improvement (Centers for Medicare & Medicaid Services, 2021). Therefore, this instrument was appropriate and ideal for use in this project. In addition, the toolkit was expected to offer insights into the variations of CRC screening.
In addition to the instrument described above, the project involved collecting data from the site’s electronic health record database. The New York hospital primary care clinic routinely collects and stores data on patients’ diagnoses and care gaps. With authorization from the site’s leaders, the PI obtained access to this data. One nurse was tasked with helping the PI to acquire and provide the data. The hospital’s information technology department personnel also provided support and assisted the PI with ensuring that the data collected was in an easily readable and manipulable format. The data still resides within the hospital’s system, with access only granted to individuals directly involved in the project. Since the hospital already has procedures for securing the data, confidentiality is continually being guaranteed and maintained. For instance, the hospital restricts access, has implemented a stringent password policy, and backs up all data to a secure cloud platform.
Furthermore, all personnel, including the PI, one nurse, and the IT department, have received updated data safety and management training. This training helped in collecting de-identified information and helped determine the number of participants in the group that had been screened, and who had developed CRC during the project’s period. In summary, while the HEDIS measure made it possible to determine the impact of the project, the data from the site’s systems allowed for the specific effect of screening using the SATIS-PHI/CRC toolkit had on screening rates among the participants.
As established above, HEDIS was the primary tool used for data collection. Essentially, HEDIS allowed for the impact of the project to be determined by tracking CRC screening rates among the participants taking part in the project. The specific data gathered using this tool include the CRC status, the particular screening method used, and the screening dates for the patients. The hospital was involved in data collection and documentation during the entire project. Basically, at the beginning of the project, the CRC status of the participants was recorded in the hospital’s electronic health record database. A similar record was entered into the system at the conclusion of the project. With authorization from the hospital’s authorities, the records were retrieved to establish differences in the screening rates at the project’s beginning and end.
The SATIS-PHI/CRC toolkit was essentially the intervention used to undertake the screening, and it guided how the screening was conducted and was not used for actual data collection. Among other things, this toolkit made it possible to ensure that the screening was undertaken in full compliance with established best practices and guidelines. Furthermore, thanks to this toolkit, the participants were enlightened on the role of screening in preventing CRC.
Validity
When undertaking their project, investigators are expected to ensure that the conclusions that they reach are valid. For the current project, validity was secured through a variety of measures. Among these measures was the use of data collection instruments whose validity has been proven through extensive research. For example, as the National Committee for Quality Assurance (NCQA) determined, HEDIS measures are among the valid tools that allow practitioners to monitor CRC screening reliably. Essentially, this instrument measures whether a patient has been screened for the condition and is, therefore, a solidly valid instrument. Scholars such as Paita et al. (2003) determined; that the HEDIS measures are valid and reliable. The Cronbach’s alpha for this toolkit has been calculated as 0.88, suggesting that it possesses acceptable internal validity (Lied et al., 2002).
In a study to test the validity of HEDIS measure sets, an analytic framework postulates a statistical relationship between health plan structural characteristics and enrollment composition was used (Paita et al., 2003). Three broad categories of health plan performance, process, objective outcome, and subjective outcome, were used to demonstrate such a relationship (Paita et al., 2003). Process refers to health care services provided or medical interventions applied. Objective outcome refers to enrollees’ health condition or health status. Subjective outcome refers to plan performance measured qualitatively (Paita et al., 2003).
To determine the content validity of HEDIS measures which is the extent to which a set of HEDIS measures includes the universe of factors that should have been included to evaluate a health plan’s performance, the relationship between a managed care plan’s structural characteristic and the composition of its enrollment is compared with the plan’s process measures. In addition, process measures and outcome measures are compared to determine HEDIS’s predictive validity, the extent to which a set of HEDIS measures predicts an outcome or set of outcomes. Finally, process measures and subjective performance measures are compared to determine HEDIS’s attributional validity, the extent to which the variation in enrollee satisfaction can be attributed to enrollment mix and structural characteristics instead of health plan performance. HEDIS was found to be valid and measures what it set out to measure.
Along with the HEDIS measure, data collection systems that the site already has in place served as a vital source of data collected for the project. For example, among the details that the PI collected were the actual CRC-related diagnoses of the patients whose healthcare needs are addressed at this facility. This data is deemed valid, purposeful, and insightful. In addition, the EPIC electronic health record system has been tested and found to be an accurate and reliable source of healthcare information. CHI St. Luke’s Health/Baylor St. Luke’s Medical Center and UT-MD Anderson Cancer Centre in Houston, Texas, performed a 2016 systematic and comprehensive review of the scientific literature regarding EPIC’s Electronic Health Record System advantages and disadvantages in terms of “meaningful use” (Johnson, 2016).
The review found that EPIC excels in providing accurate/connected information virtually in real-time to adjust medical practice. It also found that EPIC enhances patient safety, monitoring, tracking, continuity of care, and patient involvement and is a promise as a medical education tool (Johnson, 2016). The report concluded that EPIC provides a high-quality, tech-savvy front-end-to-back-end electronic health record (EHR) database for collecting and managing accurate “raw” inter-connected medical record data for timely reporting (Johnson, 2016). However, it is worth noting that researchers who have examined the validity of EPIC have not necessarily provided concrete figures that can be generalized. Instead, they focused on how reliable EPIC systems are for documenting and storing data relating to specific populations (Altman et al., 2018; Knake et al., 2016).
Reliability
In addition to using valid data collection instruments, the PI also confirmed that the tools are reliable. Reliability essentially assesses how well an instrument delivers replicable results consistently. The HEDIS measures instrument is deemed reliable and has received the endorsement of various scholars who concur that it is an essential tool for administering CRC screening (Paita et al., 2003). The analysis of the reliability of HEDIS measures assesses the procedural reliability and the internal reliability of HEDIS measures (Paita et al., 2003). The procedural reliability assessment examines the consistency of HEDIS measures across different measurement methods by comparing the HEDIS measures collected by three independent organizations for the same health plans (Paita et al., 2003).
To assess internal reliability, the extent to which conceptually related HEDIS measures indicate similar levels of health plan performance, the correlation between specific measurements within comparable measure sets was examined (Paita et al., 2003). Paita et al. (2003) found that strong agreement on HEDIS measures for the same set of health plans indicated high procedural reliability. The researchers also found that high internal reliability was observed within individual plan’s HEDIS reports, such that health plans that perform well according to one measure tend to perform well based on other measures in the same set (Paita et al., 2003). The NCQA lauds that 191 million people are enrolled in plans that report HEDIS results, and that HEDIS measures assess six domains of care: effectiveness of care, access/availability of care, the experience of care, utilization and risk-adjusted utilization, health plan descriptive information, and measures reported using electronic clinical data systems (NCAQ, 2021).
That makes HEDIS one of healthcare’s most widely used performance improvement tools. Like the HEDIS measures instrument, the EPIC electronic health record (EHR) system was found reliable. In a 2013 study to determine whether a custom computer program could improve the extraction and accuracy of key outcome measures from progress notes in an electronic medical record, compared to a traditional data recording system for incontinence and prolapsed repair procedures, Steidl and Zimmern (2013) concluded that the disciplined use of EPIC’s electronic medical record (EMR) template was vital to extracting accurate information. The researchers note key outcome measures in that data extraction improved pre-post-EPIC 53.3% to 91%, respectively, and accuracy was nearly 100%. They deduced that EPIC was the ideal front-end-to back- end interface between required documentation and clinical research (Steidl & Zimmern, 2013). In a separate study that discussed pitfalls, challenges, and benefits experienced in transitioning to an integrated electronic health recording system, researcher Bornstein (2012) concluded that EPIC far exceeded (non-meaningful) traditional paper and electronic records management systems (Bornstein, 2012).
Data Collection Procedures
The HEDIS measures are among the instruments deployed as part of the project. The HEDIS were intended to monitor and track the success of the intervention (the SATIS-PHI/CRC toolkit) and the project’s outcomes. In particular, the HEDIS measures helped determine the intervention’s effectiveness as measured by the changes in screening rates pre-and post-intervention, shed light on the patients’ experiences of care, and evaluated the access by patients to the intervention. The most recent version of the HEDIS Reference Guide for Primary care outlines some steps on how facilities and care providers can implement best practices and improve performance in colorectal cancer screening (Medicare Advantage Plans, 2021).
Among other things, the dataset stipulates that in addition to using such screening protocols as colonoscopy and computed tomography, the providers should also engage in patient education while ensuring that patients have a range of screening options available (NCQA, 2016). For example, patients who object to colonoscopy should be provided with a less invasive screening approach. Overall, the toolkit outlines various strategies that medical facilities should adopt to ensure the success of CRC screening programs. The standards that the HEDIS measure highlights informed how the project’s site implemented the AHRQ’s SATIS-PHI/CRC toolkit. For example, the implementation was evaluated based on the screening options available to the participants, the impact of the SATIS-PHI/CRC on screening rates, and whether any safety incidents resulted in adverse outcomes.
It should be noted that the HEDIS measure was not administered to the patients or the practitioners taking part in the project. Instead, it was used to assess the entire project by considering the outcomes. For example, it was held that if the screening using the SATIS-PHI/CRC toolkit resulted in a significant increase in screening rates, the HEDIS standards would suggest that the project had accomplished its purpose. Furthermore, HEDIS was employed in assessing whether the site implemented the recommended screening protocols and ensured that the patients’ preferences were honored.
On the other hand, data was collected from the site’s electronic health record database, which holds such information as the CRC screening status of the patients. The SATIS-PHI/CRC toolkit was the instrument that was used to conduct the actual screening. Since the tool is relatively comprehensive and extensive, its application to the site was limited to the six-step process that it outlines. The first step involves recruiting providers to participate in the project (Harris & Borsky, 2010). Here, the New York hospital was identified as an ideal site given the large number of older adults it serves and the absence of a robust CRC screening program. Next, the SATIS-PHI/CRC toolkit advises that academic detailing should be performed (Harris & Borsky, 2010). This step aims to introduce the practitioners involved in the implementation to the various issues they should understand. For example, as part of the project, the site’s nurses received literature that addressed CRC screening best practices to prepare them for the project.
Step three involves the identification of the eligible patients (Harris & Borsky, 2010). In following this step, a clear inclusion criterion was developed. The main requirement that patients needed to meet to be eligible to participate was aged between 50 and 75 years. The fourth step required screening information and other materials to be mailed to the participants (Harris & Borsky, 2010). During this phase, all eligible participants were invited to participate in the project, with the nurses at the site holding in-person conversations with their patients about the possibility of becoming involved in the project. In step five of the process, the toolkit advises that the provider track the screening and keep meticulous records of the results obtained (Harris & Borsky, 2010). Lastly, the toolkit advises that the provider be issued feedback based on the project’s results to inform future responses to CRC.
Due to the COVID-19 protocols in place and because the current project was a quality improvement project, the PI did not directly contact the participants. Instead, the PI gathered information from the project site’s electronic health record database. Lastly, four weeks after the project’s completion, the PI reached out to the site management for assistance to gather information regarding the screening rates. To guarantee data security and protect the integrity of the site’s data system, the PI only sought de-identified information on the CRC screening status of the participants involved in the project.
As one of the main criteria for the project, participants must be between 50 and 75 years old. The convenience sampling method made it possible to recruit the n=100 participants. Furthermore, the minimal inclusion criteria helped to broaden the population that expressed interest in taking part. Implementation of the intervention was conducted using the SATIS-PHI/CRC toolkit.
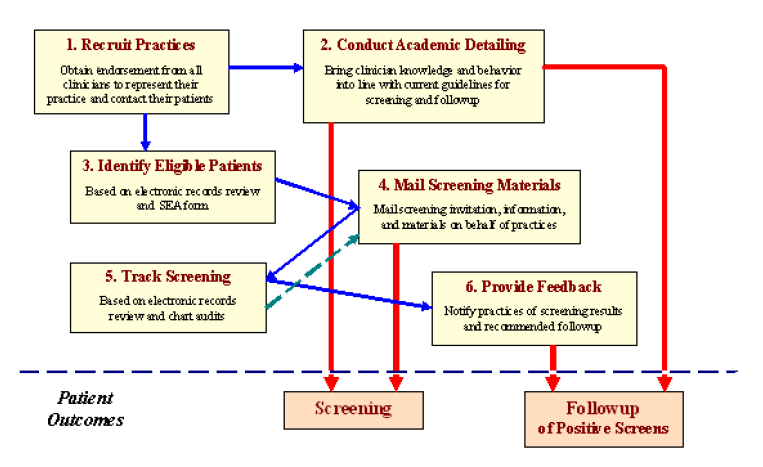
The Intervention and Assessment below were followed according to the System Approach to Tracking and Increasing Screening for Public Health Improvement of Colorectal Cancer toolkit framework (AHRQ, 2018a).
- Recruit practices,
- Conduct academic detailing.
- Identify eligible patients.
- Mail screening materials.
- Track screening.
- Provide feedback.
- Conduct assessment.
First, the PI reviewed the literature to ensure that the project benefited from clinical knowledge. Among the issues that the literature addressed include the benefits of screening, the challenges that practitioners usually encounter when determining the correct screening procedure for the right patient, and screening best practices. Secondly, eligible patients were identified by developing inclusion criteria that allowed all patients treated at the site and aged between 50 and 75 to participate in the project. The patients received mailed surveys that were designed to establish if they satisfied the inclusion requirements. Once the project was underway, the electronic health record system at the hospital was relied on for data on the CRC status of the participants. During the entire project, the PI maintained close communication with the hospital. At the end of the four weeks, an assessment of the entire project and the screening, in particular, was performed to determine the outcomes using the HEDIS measure.
The personalized screening that the participants underwent was rigorous and extensive. Each patient had a one-on-one session with the practitioners during which they received further education on CRC and the role that screening plays in preventing the occurrence of this condition. After confirming that each patient had fully understood how the screening would be conducted, the practitioners outlined the various screening approaches, including, but not limited to, fecal occult blood test, colonoscopy, and computed tomography. As standard, the hospital routinely uses fecal tests and colonoscopy to screen for CRC. However, during the project, the participants were invited to select their preferred screening methods as the practitioners understood that some patients might be reluctant to undergo invasive procedures such as colonoscopy.
Therefore, the practitioners kept detailed records of the specific screening protocol used for each patient. These records were maintained in the hospital’s electronic database to ensure security and protect patients’ privacy. At the end of the session with each patient, the practitioners provided further education on the various measures that they can adopt to reduce their risk of developing CRC. Essentially, the redesigned screening process that the patients received was personalized in full compliance with the stipulations of the SATIS-PHI/CRC toolkit. In addition to consulting the participants regarding their preferred screening method, the practitioners also conducted patient education that responded to each patient’s unique needs and concerns. The screening took place over four weeks, after which each patient’s CRC status was established.
The screening protocol described above replaced a program that the hospital already had in place. Previously, the hospital committed little effort to encourage older patients to be screened. Practitioners left it up to the patients to approach them for screening. Most of the screening cases at the hospital involved patients who had already begun exhibiting symptoms or had been referred from other facilities. Over the four weeks during which the project was implemented, screening for CRC became more routine, and practitioners became more active in the process.
Once the implementation was completed, information on screening was collected four weeks after. A manual chart review was conducted, and data were collected using the HEDIS measure instrument. Data was also collected from the System Approach to Tracking and Increasing Screening for Public Health Improvement of Colorectal Cancer toolkit survey response. HEDIS measures identify opportunities for improvement, monitor the success of quality improvement initiatives, track progress, and provide a set of measurement standards that allow comparison with other plans. The HEDIS measures also provided the identification of performance gaps and the establishment of realistic targets for improvement.
The PI undertook measures to protect the participants’ rights during data collection while avoiding or minimizing bias. For example, in the mailed-out materials, the PI explained the project’s purpose to all the participants and assured them that the project did not carry the risk of harm. Moreover, participants were told that they had the freedom to withdraw from the project at any time and were not required to explain their rationale for withdrawing. This step was designed to eliminate coercion.
In addition to protecting all digital data related to the project using passwords and encryption, the investigator also ensured that no other individual had access to this data. The data was collected from the site’s EPIC electronic health record database pre-and post-intervention implementation. This data included participants’ age, as one of the main criteria is for each participant to be between aged 50 and 75. Data were collected on the participant’s colorectal cancer screening status and colorectal cancer diagnosis status. Information was also collected on the ethnic background/race and gender to help explain and put the project result in perspective.
Data were collected at the start of the project and four weeks after the intervention was implemented. The data collected were de-identified with the help of the site’s floor manager and the tech department. Audit of information was done to check for data accuracy and then transferred to a spreadsheet where the PI inputted quantitative data in a password-protected locked computer system at the site for analysis. A backup copy of the data was secured on a flash drive and placed in a drawer compartment at the project site locked with a combination key accessible only to the PI. This was done to prevent data loss. Once the data was entered into the locked password-protected computer and on the flash drive, all paper charts/information was discarded in the locked, confidential box located in secure sites in the clinic. The PI will delete the data three years after the project concludes.
In summary, the intervention involved a redesign of the screening process using the SATIS-PHI/CRC toolkit. Over the four weeks, the 100 participants underwent screening performed with the assistance of nurses and other practitioners at the project site. The site maintains an electronic record that contains such details as the screening status of the patients. At the conclusion of the project, the HEDIS instrument was used to evaluate the intervention. The HEDIS measures proved vital as they allowed for the overall outcomes and impact of the SATIS-PHI/CRC toolkit to be determined.
Data Analysis Procedures
The main clinical question that the current project sought to answer was whether a redesign of screening using the System Approach to Tracking and Increasing Screening for Public Health Improvement of Colorectal Cancer toolkit effectively increases the rate of CRC screening at the urban primary care clinic over four weeks. Among the data collected included the age, the CRC screening, and diagnosis status of the participants. The raw data was organized in tabular form to prepare it for rigorous analysis. The primary test used to analyze the data collected for the project was the chi-square test. This test is a statistical process used to help determine if there are any significant differences in pre and post intervention. For example, data collected on CRC screening rates before implementing the System Approach to Tracking and Increasing Screening for Public Health Improvement of Colorectal Cancer toolkit (SATIS-PHI/CRC) were measured against the data collected post-intervention implementation.
The PI analyzed the data to discover the differences using a chi-square test to help develop the hypotheses. The chi-square test was used to compare the predicted and the observed values under the selected study design. The test’s purpose was to determine whether the proposed intervention had any significant effects on disease detection after the intervention was done. The PI used Statistical Package for Social Sciences (SPSS) software for data analysis. Essentially, the PI computed the mean screening rates among the participants. A significant variance in the mean would be taken as an indication that the AHRQ’s SATIS-PHI/CRC toolkit effectively increased the screening rate, as this was the purpose of the project. In addition to chi-square test, the PI also used descriptive statistics as part of the data analysis process. For example, this approach made it possible to determine the percentages of the patients who underwent screening and who developed CRC after screening.
The data analysis techniques described above were appropriate because they serve the proper purpose. For example, the chi-square test may be used to forecast the effects or impact of changes. This may be used to identify the strength of the independent variable(s) effect on a dependent variable. Similarly, descriptive statistics is justifiable because this method offers essential summaries of the population in question. More importantly, the two analysis methods are fully aligned with the project’s design and purpose. They made it possible to determine the impact of CRC monitoring/ screening on the incidence of the disease. During the analysis, the level of significance α was set at 0.05.
Potential Bias and Mitigation
Bias can occur intentionally or unintentionally at any phase of a project and can lead to problems with its validity. When biases are mentioned in quantitative methodology, they refer to any external or extraneous factors that may interfere with the project’s findings. While measures were taken to guarantee the accuracy and integrity of the data, it is important to acknowledge that errors may have tainted the data. A possible source of the errors may be the sampling approach used. The quota sampling method was adopted for the project. Although it is cost-effective, time-saving, and relatively simple to implement, this sampling technique lacks statistical rigor and can yield errors. For example, due to quota sampling, the sample that took part in the project did not necessarily represent the larger population. New York is a cosmopolitan and tremendously diverse area. However, the sample only included individuals from a handful of racial backgrounds in one urban primary care clinic.
Confounding bias is yet another possible source of errors. Essentially, this bias occurs when individuals fail to account for factors that affect the phenomenon under investigation. For example, for the project, the CRC screening rate was the primary variable explored. The SATIS-PHI/CRC toolkit was the intervention implemented to influence this dependent variable. However, the project did not accommodate CRC incidence being a complex issue affected by a wide range of other factors. For instance, one’s socioeconomic background and educational attainment are some of the issues that shape their risk of developing and dying from CRC.
Therefore, it will be challenging to assert that the results of the project are representative and conclusive. For example, even if determined that the post-screening incidence was lower for the entire sample, it would be difficult to conclude that the participants’ experiences were the direct and exclusive result of the CRC screening they undertook. The best strategy for improving the project’s internal validity was using a research design with control mechanisms such as instrumentation and participant selection. Polit and Beck (2017) noted that the pre-test and post-test approaches could create a weakness as the pre-test may affect the post-test outcomes. The failings may be attributed to the familiarity with the test (Sylvia & Terhaar, 2014).
Data analysis may also have a bias. If there are missing data or errors with data entry, this may contribute to bias (Sylvia & Terhaar, 2014). The primary investigator guaranteed that all findings were accounted for and completeness of all surveys used in the project. Additionally, with the lack of randomization for participants’ selection for the sample, the risk of selection bias was present. Consecutive sampling would have less probability of bias than convenience sampling (Polit & Beck, 2017). Further issues with potential bias are that participant selection was voluntary and might represent an inaccurate population depiction and the limited sample size used in the project.
Ethical Considerations
Several policies are designed by authorities to promote and protect the integrity, compliance, and ethical standards in the way research and direct practice improvement projects are conducted. Deception in a project challenges the quality of establishing evidence-based interventions that should be put in place to prevent unethical practices (Yip et al., 2016). A general overview of ethical and legal principles enables researchers to research according to best practices (Yip et al., 2016). During the entire project, great care was exercised to adhere to the established ethical standards. The PI gave particular attention to ensuring the safety and wellbeing of all the participants.
During the project, many ethical issues could have arisen. They included obtaining informed consent, protecting participants against harm, and guaranteeing participants’ privacy and confidentiality while avoiding conflict of interest. These issues can undermine a project and expose participants to the risk of adverse outcomes if left unaddressed. In response to the ethical issues, the PI instilled several measures to protect the project. For example, although informed consent was not necessary, the PI explained the project’s purpose and ensured that the participants understood the risks and voluntarily agreed to participate in the project. In addition, the PI included this information in the flyers that all the participants received.
On the other hand, privacy and confidentiality were achieved by encrypting data and using code names to refer to the participants. Furthermore, no participants were required to provide any personally identifiable information such as their name or address. Additionally, the PI worked with the site to anonymize all data during the data analysis process.
Another measure that the PI adopted was to obtain approval from the institution’s quality improvement (QI) officer. The designated site is not involved in research and requires the QI officer to sign off on DPI and QI projects. The PI put all measures to ensure full compliance with all relevant ethical requirements and considerations to gain approval; furthermore, the project adhered to the principles of the Belmont Report. Commissioned by the U.S. government in response to ethical failures, the report sets ethical standards for research and proposes three principles that should underlie ethical conducts of research involving human subjects: Respect for persons, beneficence, and justice (Sims, 2010). These three principles, which are somewhat abstract in the report, were later operationalized into the detailed rules and procedures that make up the Common Rule, which governs research at U.S. universities (Montgomery, 2021).
Limitations
While the project is robust, it suffered some limitations. At only 100, the sample size is small given that thousands of individuals in the U.S. are at risk of developing CRC. This limitation is unavoidable given the circumstances under which the project was conducted. For example, the COVID-19 pandemic meant that it would be unacceptably irresponsible to recruit a large number of participants who would be forced to interact with each other and healthcare professionals at the site, thereby increasing the risk of contracting COVID-19. Given the age of the participants, COVID-19 would result in severe illness or even death. Another shortcoming of the project is that it took place in New York, an area whose population does not necessarily represent the general American population. The PI selected the site because of convenience and practicality considerations. It would be difficult for the PI to complete the project farther away. Also, for the hospital’s urban primary care clinic, there are 11 in the network, yet the project only included a sampled population from one.
Despite the limitations described above, the PI followed all protocols for the project to ensure that the project had rigor and validity and did not erode its quality. For example, the small sample size was still sufficiently large to enable the PI to examine how screening influences CRC screening rate. The choice of area hosting the project did not adversely affect the quality of the project because the PI acted to ensure that the sample was an accurate representation of the population the urban primary care clinic serves. Based on the ethnic background of the selection, it was realized that it depicted the make-up of the clinic’s population.
Despite the limitations and delimitations outlined, the PI instituted safeguards to protect the validity and reliability of the project. For example, the PI used valid and reliable data collection instruments. Furthermore, the PI exercised caution to ensure that the participants’ rights were guaranteed. The QI/DPI officer approved the project, and the university’s IRB approval was granted before the project’s initiation.
Summary
In closing, this chapter has offered a detailed description of how the PI carried out the project to satisfy the project’s intent. The project’s goal was to establish whether a redesign of the screening process using SATIS-PHI/CRC would impact CRC screening rates in an urban New York primary care clinic. The project involved a group of 100 participants aged between 50 and 75 to achieve this objective. The group underwent CRC screening using the AHRQ’s toolkit intervention. Pre and post-test implementation of intervention and data collected were analyzed and compared and revealed whether the intervention helped increase the screening rate of CRC.
The PI instituted data collection instruments and procedures for the project. HEDIS measures facilitated the collection of data on the CRC screening status of the patients. The PI used two appropriate techniques for the project, the paired t-test and descriptive statistics were used to analyze the data,
In the following chapter, the focus of the report shifts to an exploration of data analysis procedures and the results obtained by the investigator. The fourth chapter addressed the specific issues included the sample characteristics and observations regarding the impact of early CRC screening. Finally, chapter four outlined the findings of the project. The purpose of Chapter Four was to determine if the methodology used yielded vital insights.
Data Analysis and Results
The purpose of this quantitative quasi-experimental quality improvement project was to determine if or to what degree the implementation of AHRQ’s SATIS-PHI/CRC toolkit would impact colon cancer screening rates when compared to current practice among patients aged 50-75 in a primary care clinic in urban New York over four weeks. As part of efforts to accomplish this purpose, the project examined if and to what degree a redesign of the CRC screening process using the Agency for Healthcare Research and Quality CRC screening toolkit at an urban New York primary care clinic would cause a sustainable and significant change in the rate of CRC screening. In addition, by allowing practitioners to detect CRC early through screening, the AQHR’s toolkit could make it possible to reduce CRC incidence rate by creating opportunities for the participants to receive early treatment for any precancerous lesions or polyps or other ominous signs of CRC detected during the screening procedure. Therefore, a quantitative quasi-experimental approach that involved a sample of 100 participants was adopted for the project.
This chapter focused on the outcomes of the project. The chapter discussed and outlined the results obtained following the implementation of the intervention for the project. The topics covered include both descriptive and quantitative data that the project yielded and the procedures employed in the data analysis. The purpose of this chapter was to determine if the results of the project confirmed whether there was an increase in CRC screening rates.
Descriptive Data
The project involved a sample of n=100 participants from different ethnic backgrounds. This sample was intended to represent the DPI site’s population and is in the 50–75-year age bracket known to be among the most at risk for CRC. Therefore, as part of the data analysis process, the sample was examined for its composition. Among the demographic features that formed part of the analysis was the racial/ethnic background of the participants. The table below illustrates the racial identities of the participants.
Table 1. Colorectal Cancer Screening Demographics.
The sample was representative of the population that the hospital’s primary care clinic hosting the project serves. A large majority of the local population is Latino (60%), with African Americans accounting for a significant portion (18%). On the other hand, Caucasians are a minority within this community, making up just 6% of the total population. Such other groups as Native Americans, and Asian Americans, account for the rest of the community. The community is primarily young, with individuals aged between 16 and 35 comprising about 30% of the total population. On the other hand, older adults are a minority. Recent census data showed that just 8% of the area’s population are senior citizens aged 65 and above.
Apart from ethnicity, age was another identifier by which the sample was analyzed. All participants were aged between 50 and 75. All individuals needed to be in this age group, to be included in the project. The following graph shows the age distribution of the sample.
A power analysis was conducted using G*Power to determine the required minimum sample size for the project. Four factors were considered in the power analysis: significance level, effect size, the power of the test, and statistical technique. This is how the PI arrived at a sample size of 100. The significance level, also known as Type I error, refers to the chance of rejecting a null hypothesis, given that it is true (Bhandari, 2021). Most quantitative studies use a 95% confidence level because it adequately provides enough statistical evidence of a test (Creswell & Poth, 2017). The effect size refers to the estimated measurement of the relationship between the variables being considered (Cohen, 1988).
Cohen (1988) categorizes effect size into small, medium, and large. Gong and Xie (2019) purported that a medium effect size is better because it balances between being too strict (small) and too extensive. The power of the test refers to the probability of correctly rejecting a null hypothesis (Sullivan & Feinn, 2012). In most quantitative studies, an 80% power is usually used (Sullivan, & Feinn, 2012). The statistical test used for this project was the chi-square test. A power analysis was conducted to establish the appropriate sample size for the project. The goal of the power analysis was to determine the appropriate size of the sample to ensure that the project’s findings could be generalized to the larger population.
To conduct a chi-square test to detect a medium effect size, at the 5% level of significance, with 80% power, at least 800 participants are required. Assuming that the project satisfies the minimum requirement of a power value of 80%, the PI would be required to recruit more than 800 participants, far much higher than the 100 participants recruited for the project. However, following lengthy discussions with the site’s authorities, it emerged that a project with 800 participants would be infeasible for various reasons. First, the hospital made it clear that the total number of patients aged 50-75 who were receiving treatment at the time was less than its normal capacity due to the coronavirus constraints. Multiple patients had relocated due to the virus and several staff were out sick. These facts implied that the ideal sample size could not be achieved. Secondly, the hospital faced severe financial and other constraints that undermined routine care delivery when the project was conducted. Therefore, requiring the hospital to screen 800 patients would have stretched its already thin resources even further.
Thirdly, as noted earlier, the project was conducted over four weeks. Given the time limitation, it was not possible to screen 800 individuals. These factors were considered critically, and after further consultations with the hospital’s leadership, it was determined that the hospital could screen 100 individuals over the four weeks without compromising other operations and patients care. Convenience, costs, and practicality are the main factors that were considered when setting the sample size at n=100. Basically, in consultation with the authorities at the site, the PI determined that a sample of 100 was large enough to collect credible and high-quality insights while still ensuring that the project could be practically conducted. While practicality and cost are the primary considerations that informed the sample size, previous research was also consulted when determining the number of patients to include in the project. For instance, according to Brysbaert (2019), whereas a sample should be as large as possible, a sample size of 100 is acceptable as it meets basic power requirements. In addition, the analysis made data possible from the incidence rate of CRC in New York City.
On the one hand, data regarding the CRC incidence rate for Bronx County (the site’s location) was obtained from the New York State Cancer Registry (2021). According to the registry, the average CRC incidence rate for adults aged 50 and 75 is 69.44 per 100,000. On the other hand, the average national incidence rate was 38.7 per 100,000 (Siegel et al., 2020). Using these figures and setting the Alpha at 0.05 and the power at 90%, it was determined that the sample would need to have at least 25 participants for the project to be credible. The local CRC incidence rate is near twice the national average, and the local CRC incidence rate can explain the small sample size.
Therefore, only a small number was needed to determine if the patients involved in the investigation would represent the larger population. However, to guarantee the reliability and validity of the project’s findings, it felt wise to increase the number of participants to 100. Increasing the sample size to 100 increased the statistical power to 99.86%, as computed by G*Power post hoc sample size calculations. This sample was determined after consultations with various stakeholders at the primary care clinic at the urban primary care clinic where the project took place. These discussions revealed that at least 100 patients would be prepared to participate in the project. As stated earlier, practical and cost concerns informed the sample size. Furthermore, as already established, such scholars as Brysbaert (2019) have found 100 a reasonable sample size.
Data Analysis Procedures
At the end of the four weeks, all the participants underwent CRC screening. Furthermore, the PI also used the HEDIS measure to evaluate the overall effect and outcomes that the AHRQ’s SATIS-PHI/CRC tool had on screening in the facility. As discussed in greater detail in a later section, HEDIS is a tremendously valuable instrument that allows healthcare providers to assess the effectiveness of the solutions they put in place to improve care delivery. However, while HEDIS measures are rather extensive tools that measure various outcomes, the instrument’s use in the project was limited only to the CRC screening, a critical metrics of this instrument.
Descriptive statistics were among the procedures used to break down and dissect the data that emerged from the project. In addition to allowing the participants’ demographic profiles to be constructed, the PI used descriptive statistics to determine the CRC screening outcomes. For example, the PI used this approach to determine differences in the CRC screening rate before and after implementing AHRQ’s System Approach to Tracking and Increasing Screening for Public Health Improvement of Colorectal Cancer intervention toolkit. The descriptive statistics technique also made it possible to highlight the participation rate among the sample. All the participants took part in the entire project.
In addition to descriptive statistics, the data analysis also involved the use of the chi- square test. This test is appropriate for various reasons. First, the chi-square test allowed for the determination of the AHRQ’s System Approach to Tracking and Increasing Screening for Public Health Improvement of Colorectal Cancer toolkit as a reliable instrument for evaluating the effectiveness of screening. The PI collected data on the CRC screening rates at the beginning of the project and four weeks after introducing the intervention. Second, the paired sample chi-square test was intended to reveal any statistically significant difference in the CRC screening rates, pre, and post-implementation of the AHRQ’s toolkit.
Most importantly, the chi-square test was suitable because it allowed for the analysis of the categorical date taken during the project. After four weeks, the PI analyzed the CRC screening rates and CRC incidence. This time, the focus shifted toward determining if any of the participants had developed CRC. The paired observations (pre and post-test CRC statuses) were then analyzed using the chi-square test. Essentially, this test helped determine whether the redesign of the CRC screening process led to a significant change in CRC cases among the participants.
The independent sample t-test is among the most popular protocols for examining the participants’ experiences when participating in healthcare research or inquiry and was considered for this project. However, the independent sample t-test was deemed unsuitable for several reasons. First, this test is typically conducted to compare two different interventions. Since the project involved SATIS-PHI/CRC as the only intervention, the independent sample t-test was inappropriate. Secondly, this test is carried out when there are two separate groups of participants. This was not the case in the project, which had just one group of participants who were all provided with screening and had their CRC status established at the beginning and the end of the project.
Before selecting a chi-square test, the PI tested to determine whether data was parametric or non-parametric. The criteria involved in testing included testing for normality of the difference scores and outlier detection. Normality was assessed by the calculation of skewness and kurtosis values. As recommended guidelines, skewness values within -3 to + 3 and kurtosis values between -7 to +7 were used. The kurtosis values fell outside the normal threshold. Due to the violation of the normality assumption, the chi-square test and Wilcoxon signed-rank were performed. The Wilcoxon signed-rank test is used to determine if there are differences on a dependent variable between two related groups (Pre and post-intervention groups).
The data analysis procedures followed in the project align with the protocols for which the PI obtained approval before the project’s implementation. The question upon which the project sought to answer was whether implementation of the AHRQ’s SATIS-PHI/CRC toolkit resulted in improvement in the rate of CRC screening. All the data analysis methods worked together to compare the participants’ experiences and outcomes before and after the implementation of the intervention, thereby allowing for the relationship between AHRQ’s SATIS-PHI/CRC and CRC screening rates to be established. Furthermore, the analysis methodologies were appropriate for the quantitative quasi-experimental project’s design because the focus was on quantitative data.
Results
In the following sections, an overview of the results obtained from the project is provided. Essentially, the project sought to determine if and to what degree the implementation of AHRQ’s System Approach to Tracking and Increasing Screening for Public Health Improvement of Colorectal Cancer toolkit compared to the current practice, would increase colon cancer screening rate in an urban New York primary care clinic over four weeks?
CRC Screening and CRC Screening Rates
As is evident this far, the project’s primary objective was to evaluate the effectiveness of the SATIS-PHI/CRC toolkit as an instrument for undertaking CRC screening among patients aged 50-75 years in an urban New York primary care clinic. At the beginning of the project, only three of the 100 patients were screened for CRC before. During discussions with nurses at the facility, standardized screening was a problem as some providers were not following age-related protocols. Furthermore, the nurses noted that patients were reluctant and did not want to undergo some recommended screening procedures. It also became clear that the hospital did not have a specific program tailored for personalized CRC screening. Therefore, it is not surprising that the screening rate pre-intervention was three in the population sample of 100. This means that, of the 100 participants, only three patients had prior screening.
The screening rate was computed again at the end of the four weeks. This time, it was established that all 100 participants had been screened. All of the patients underwent either colonoscopy, fecal testing, or computed tomography. Furthermore, the participants attended all training and screening sessions. The following graph illustrates this result:
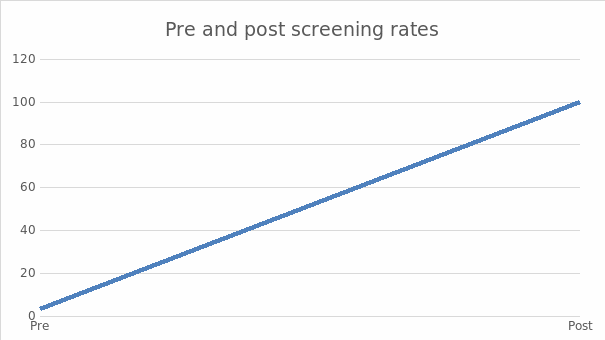
Screening rates
As the graph above shows, following the implementation of the screening intervention, there was a 97% increase in the screening rate. Therefore, the PI conducted the chi-square test to establish whether this change resulted from screening using the SATIS-PHI/CRC toolkit. The following is the summary of this test:
Table 2.Frequencies for Pre-intervention.
Table 3.Frequencies results for Post-intervention.
Table 4.Chi-square Tests.
The tables above summarize some of the most vital statistics yielded by the Chi-square test. From the table, at X (1) = 88.360, P=.000, given p<0.05, the study concludes that the intervention had a significant effect on screening rate.
CRC Screening
Descriptive Statistics
One of the project’s key goals was to establish if the implementation of AHRQ’s SATIS-PHI/CRC toolkit would significantly affect CRC screening. Previously, the primary care clinic that hosted the project struggled to persuade patients to undergo screening, especially those in the 50-75 age groups. As a result, screening rates averaged 38% over the previous three years. Before the pandemic, the rate of CRC screening was at 55%. Still, it dramatically plummeted during the heart of New York’s COVID crisis as most specialty clinics were closed. Patients were advised to stay home or away from compromised locations to prevent the spread of the disease. The project triggered a dramatic increase in this figure. All the 100 individuals who participated in the project were screened. In essence, the CRC screening rate spiked to 100% in the sample screened, indicating that the interventional program promoted screening at the facility. There is a 97% increase from the pre-intervention screening rate of 3%.
At the end of four-week, six cases of early CRC were suspected. The screening identified six participants with symptoms ranging from having two to multiple adenomatous polyps and a positive occult stool test. These six cases suggested that the proportion of positive CRC status rose by 6%. However, it should be understood that all six participants were not clinically diagnosed with CRC. Instead, two participants were diagnosed with CRC after the biopsied samples’ pathology results returned from the lab. For the others, the screening showed that they exhibited symptoms indicative of needing surveillance to ensure appropriate treatment for CRC detected in its early stages.
After all, if left untreated and not monitored, there is a strong likelihood that the adenomas polyps, which are called precancerous lesions, may develop into colorectal cancer. CRC screening allows identifying patients with symptoms that could lead to CRC development if appropriate and timely interventions are not administered. In compliance with standard practice and ethical requirements, the patients were provided with proper care in all six cases and information on the implications of the screening results offered. At no time during the entire project was their safety, well-being or identity compromised.
Above, it was noted that only six participants exhibited symptoms of early-stage CRC. After appropriate intervention to these individuals, just two met the requirements for clinical CRC diagnosis. Thus, the actual CRC incidence rate after implementation of the SATIS-PHI/CRC toolkit was 2%. However, compared to the pre-implementation baseline of the sample, which was 0, the CRC incidence rate rose 2%. This finding underscores the crucial role that screening plays in enabling practitioners to deliver appropriate treatments to patients who exhibit symptoms of early CRC and is essential. In addition, the four patients whose symptoms were resolved through treatment illustrate that CRC screening can serve as an effective CRC prevention strategy when appropriately implemented. The graph below summarizes the change in the CRC incidence rate of the clinic.
As is evident from the graph above, before implementing AHRQ’s SATIS-PHI/CRC toolkit, none of the participants had CRC. However, at the end of the four-week, two of the 100 participants had developed CRC. In itself, this finding does not necessarily mean that the screening increased the CRC incidence rate. For this reason, the Chi-square test was undertaken to determine whether the pre-and post-intervention differences in the CRC rates were statistically significant.
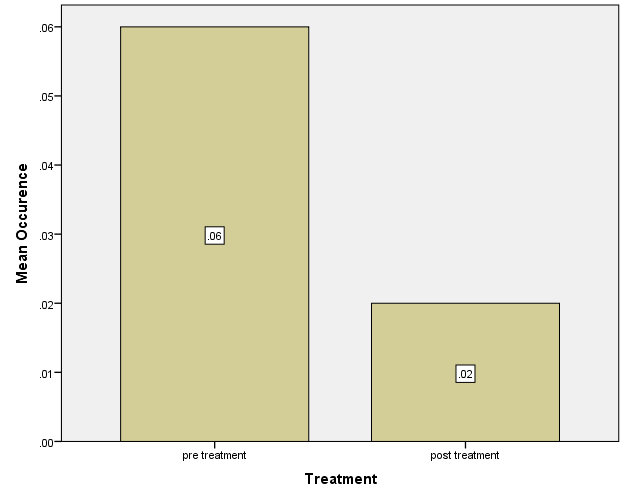
The graph above essentially underscores screening and early treatment’s role in protecting patients against CRC. During the project, six patients exhibited symptoms that were consistent with CRC. The patient received treatment that resolved the signs in all but two of these cases in line with ethical standards. After the project, the two patients diagnosed with CRC received guarantees that their cases would be closely monitored and receive aggressive treatment to manage the condition. Essentially, all effort was made during the entire project to protect the participants from harm and ensure that their well-being was secured.
Results of Chi-square Test Analysis
The PI undertook a chi-square test to determine if the observed decline in CRC incidence rate was statistically significant. However, before selecting the test, the dataset was analyzed to determine whether the parametric or non-parametric test was useful for analysis. Thus, the parametric assumption of normality of the paired differences was assessed. Normality was assessed by visual inspection of a histogram and the calculating skewness and kurtosis values. Figure 6 depicts a histogram of the paired differences from pre to post interventions. There is a huge peak with drastic dips below and above the center peak, indicating a violation of normality.
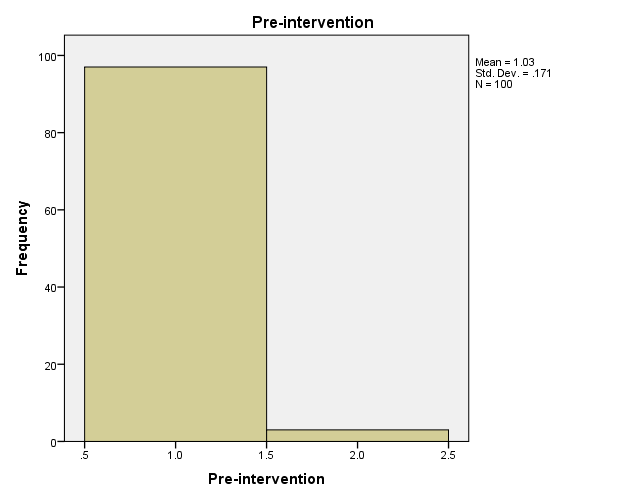
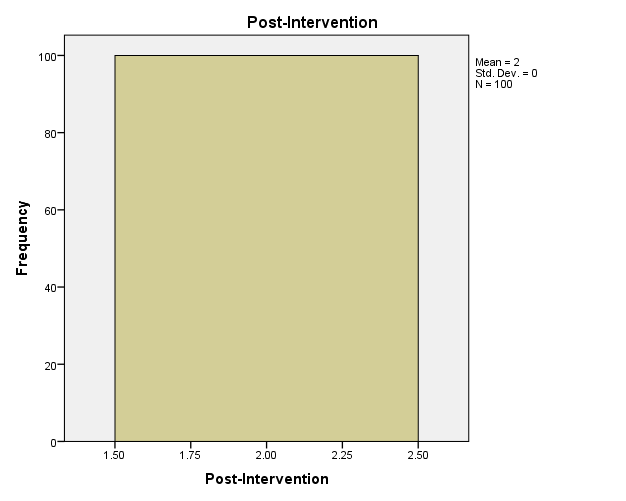
Additionally, skewness and kurtosis values were 5.595 and 29.898. Hair et al. (1998) argued that data is normal if skewness is between ‐2 to +2 and kurtosis is between ‐7 to +7. However, the value of kurtosis falls outside this range which indicates that the paired differences distribution is not normal. Due to the violation of the normality assumption, Chi-square test and Wilcoxon signed-rank test, which are non-parametric tests, were deemed vital for analysis. The Wilcoxon signed-rank test was used to determine differences in a dichotomous dependent variable (occurrence of CRC) between two related groups (Pre and post-treatment groups).
The mean screening rate in the pre-treatment group (M = 1.03) was less than in the post-treatment group (M = 2). Moreover, the standard deviation of pre-treatment groups), SD = (.171) was greater than the standard deviation in post-treatment group (SD = 0.00). A zero standard deviation and an increase the mean from pre-treatment to post-treatment suggests a 100% increase in screening rate upon the intervention. However, the Chi-square test revealed that there was no enough evidence that reveals the significant difference between pre and post-intervention results(X(1)=2.396), p=0.061). Further, as mentioned earlier, there was a violation of the normality requirement of the parametric test. As a result, the PI performed the non-parametric related samples Wilcoxon signed rank test. The Wilcoxon signed rank test was used to determine differences in a dichotomous dependent variable. The results of the analysis were as follows.
Table 5: Descriptive Statistics.
Table 6: Wilcoxon Sign Rank Test.
Table 7: Test Statistics.
A Wilcoxon Signed-Rank test showed that post-intervention screening had more number of patient who underwent screening (Mean Rank=49) than pre-intervention screening(mean rank=0,Z=-9.849, P=0.000). The results suggested that the implementation of the suggested intervention would increase the rate of screening at the facility.
Results of HEDIS Measures
The PI used the HEDIS measures to evaluate the overall project. Among other requirements, the HEDIS stipulates that when undertaking CRC screening, practitioners should administer computed tomography, fecal occult blood test, or flexible sigmoidoscopy. Since the project involved colonoscopy, computed tomography, and fecal tests, this standard was satisfied. Furthermore, HEDIS stipulates that when screening detects any cases of CRC of early symptoms, patients should be provided with treatment as soon as practicable. This stipulation was also satisfied since the patients that showed early-stage CRC signs received treatment.
Furthermore, the project involved adults aged between 50 and 75 is yet another way the project complied with the standards outlined in HEDIS. This measure recognizes that the adults in this age group are most vulnerable to CRC and must be prioritized in screening. Lastly, the HEDIS measures were relied upon to evaluate the overall impact of the SATIS-PHI/CRC toolkit by examining the changes in screening rates. Since the rate increased by 97%, one can conclude that on the whole, the project was successful.
Summary
The results of the project demonstrated that it was undertaken and completed successfully. With a 100% participation rate, the project appealed to the participants and helped them understand the crucial role of screening in protecting them against CRC. The sample that was involved in the project included participants from different ethnic, age, and gender groups. While the PI will discuss these results in detail in the next chapter, it is essential to highlight some of the most notable observations. The project demonstrated that the SATIS-PHI/CRC toolkit is an effective instrument for CRC screening. Following the administration of this toolkit, the screening rate within the participant group increased from 3% to 100%. Moreover, the project also showed that the screening process redesign using the SARIS-PHI/CRC toolkit increased the number of early-stage CRC cases. Two patients were clinically diagnosed with CRC after the six participants were provided with appropriate care to address such symptoms as precancerous lesions.
In conclusion, the project’s results showed that the intervention effectively increased screening rates in the urban primary care clinic. The data collection and analysis procedures were aligned with the clinical question: if and to what degree the implementation of the AHRQ’s SATIS-PHI/CRC toolkit compared to current practice would increase colon cancer screening rate in an urban New York primary care clinic over four weeks? The screening conducted using the SATIS-PHI/CRC toolkit resulted in changes in CRC screening. Although the results were not significant, there was evidence to suggest these relationships may be established in future studies with a much larger sample.
Furthermore, the adopted analysis protocols shed light on how issues like ethnicity and gender are risk factors for CRC. A more detailed narrative discussion of the findings and their implications is provided in the next chapter. The discussion sheds light on what the project’s results mean and the conclusions that can be drawn regarding the effectiveness of the System Approach to Tracking and Increasing Screening for Public Health Improvement of Colorectal Cancer toolkit as a tool for CRC screening for the prevention of CRC incidence.
Summary, Conclusions, and Recommendations
The focus of this chapter was to summarize the project. This chapter outlined the key findings that the project revealed using the HEDIS measures instrument and discussed the implications for practice and future research. The current project is essential. In addition to examining the role that the System Approach to Tracking and Increasing Screening for Public Health Improvement of Colorectal Cancer toolkit plays in improving screening rates and minimizing the incidence of CRC, the current project explored the burden that colorectal cancer (CRC) imposes on patients, most of who are in the 50-75 age ranges.
The project’s result revealed that the two tools (HEDIS and System Approach to Tracking and Increasing Screening for Public Health Improvement of Colorectal Cancer); are effective instruments for increasing CRC screening rates, facilitating early detection, and enabling healthcare practitioners to provide preventive care.
Furthermore, the project is crucial because it adds to the existing knowledge by highlighting how institutions can use the SATIS-PHI/CRC toolkit to increase screening rates and shield patients against CRC. Additionally, it was not previously fully understood whether a redesign of the screening process could effectively improve the CRC screening rates and reduce CRC incidence. Therefore, since the project specifically examined the impact of the AHRQ’s SATIS-PHI/CRC toolkit on CRC screening rates, it will fill an existing gap. The project was designed with a clear focus on existing literature and planned to advance scientific knowledge to ensure actual contributions to knowledge. The project involved participants whose CRC status was tracked over the four weeks to determine if the System Approach to Tracking and Increasing Screening for Public Health Improvement of Colorectal Cancer toolkit delivered the desired outcomes. Moreover, using such data collection instruments as HEDIS allowed for the generation of insights that expanded the knowledge on the dynamics of CRC and its prevention. The following sections provide an overview of the project and its contributions to theory and practice.
Summary of the Project
The purpose of this quantitative quasi-experimental project was to determine if or to what degree the implementation of AHRQ’s SATIS-PHI/CRC toolkit would impact colon cancer screening rates when compared to current practice among patients aged 50-75 in a primary care clinic in urban New York, over four weeks. This project evaluated the main issue, whether a redesign of the screening process using the SATIS-PHI/CRC toolkit would cause an increase in CRC screening. The CRC screening status of the 100 participants was recorded at the beginning of the project; four weeks later, the PI repeated the test to establish the post-intervention status and the SATIS-PHI/CRC toolkit’s impact on screening rates. Pre-and post-implementation tests outcomes were compared to determine the project’s impact on screening rates.
Several techniques were used for data analysis. The paired sample t-test was the primary protocol incorporated into the data analysis process. This test made it possible to determine if the pre-and post-project implementation differences in screening rates were statistically significant. Data analysis also involved descriptive statistics in generating such insights as to the demographic composition of the sample. Overall, the implementation of the project was successful as it yielded significant results that will inform future projects, healthcare practice, and research.
Perhaps the most important finding that the project yielded is that using the SATIS-PHI/CRC toolkit increases the screening rate. Only 3 of the 100 participants had been screened for CRC when the project began. The low screening rate may be blamed on reluctance among patients and the lack of a dedicated program for screening at the site. However, at the end of the four weeks, all participants were screened for CRC, with only two being found to have developed the condition. Thus, the SATIS-PHI/CRC toolkit played an essential role in helping the site increase its screening rates and contain the CRC incidence.
The project conclusively showed that the SATIS-PHI/CRC toolkit significantly increases CRC screening rates. Essentially, following the screening protocol at the site, there was a statistically substantial increase in the number of identified cases. Thus, it is reasonable to argue that without redesigning the screening process using AHRQ’s SATIS-CRC/PHI toolkit, the four patients who exhibited symptoms would have developed CRC as they would not have received timely treatment that could prevent this condition. Furthermore, the intervention allowed the site to design an elaborate follow-up and monitoring exercise that allowed the two patients with CRC to receive their needed care.
Summary of Findings and Conclusion
The following section summarizes some of the most important findings and conclusions from the literature and the project.
Effectiveness of SATIS-PHI/CRC for Detecting CRC and Increasing Screening Rates
The project’s primary purpose was to establish whether one can reliably use the System Approach to Tracking and Increasing Screening for Public Health Improvement of Colorectal Cancer toolkit to increase CRC screening rates while preventing CRC among adults aged 50 to 75. After the project, it was realized that 100% of the sampled population was screened. This means that the screening rates jumped from 3% pre-intervention to 100% post-intervention. Moreover, the project has accomplished its key objective by establishing that the System Approach to Tracking and Increasing Screening for Public Health Improvement of Colorectal Cancer toolkit is a reliable resource for increasing CRC screening rates. The analysis of the impact of using the SATIS-PHI/CRC toolkit to redesign the screening process showed at X (1) = 88.360, P=.000, suggesting a statistical significant effects of the intervention on the target population. Moreover, Wilcoxon Sign Rank test indicated that the toolkit improved screening rates and patient outcomes, and healthcare facilities should adopt the model.
Additionally, an increase in the CRC cases among the 100 participants sampled was observed after the project. Before implementing the project, the hospital recorded a CRC prevalence rate of 4%, while the sample’s baseline was 0%. At the beginning of the project, none of the participants taking part were found to have a diagnosis of CRC. However, after the project, the number of cases had risen to 6 in the sample, or so it seemed. It should be understood that of the six suspicious cases, four patients merely displayed signs consistent with needing CRC surveillance. For example, signs that these patients exhibited included precancerous lesions or adenomatous lesions, among others.
When the symptoms were identified, the primary investigator (PI) collaborated with the hospital staff to ensure that the appropriate measures were placed for the PCP and gastroenterologists to follow these patients. However, because the PI collected de-identified data, the PI could not identify these participants. Instead, all providers were encouraged to follow their patients based on their recent test results. The floor manager informed the PI that the patients were eventually declared free of CRC. On the other hand, the other two participants were diagnosed with CRC.
The Chi-square was carried out to determine whether the observations outlined above were statistically significant. This test revealed that the pre-and post-project implementation CRC screening rates were not statistically distinct for CRC incidence at X (1) = 88.360, P=.000). However, although not statistically significant, the result may be clinically significant. This result is crucial because it suggests that the decline in CRC prevalence at the hospital was not necessarily due to some extraneous factor. Instead, implementing the AHRQ’s toolkit allowed early-stage CRC detection and lowered CRC cases.
It was hoped that the results of the project would expand existing knowledge. As noted previously, there does not appear to be much research that focuses specifically on how screening prevents CRC. Another important implication of the result stated above is that the project mainly aligns with the expert’s insight. For example, the American Cancer Society (2017) is among the professional organizations that have held that in addition to being practical, screening is also reasonably easy to incorporate into practice as it is affordable. During the implementation of the project, only minimal resources were needed. The project’s low cost confirms that screening is cost-effective and can be integrated into a wide range of practice settings.
In addition to the issues outlined above, the project’s main result also underscored the crucial function that training serves in CRC prevention. Before the PI undertook the project, all practitioners who participated received training on such issues as how to use the System Approach to Tracking and Increasing Screening for Public Health Improvement of Colorectal Cancer toolkit. There is reason to believe that the project would not have accomplished its purpose without the training, and the patients may have suffered some harm. Therefore, as they incorporate the AHRQ’s SATIS-PHI/CRC toolkit in redesigning their screening process, medical institutions should recognize the value of rigorous and ongoing training for their personnel.
Implications
The project’s results present numerous implications, some of which are highlighted in the following sections. First, the effectiveness of early preventive care in screening exams cannot be understated. In increasing the rate of colon cancer screening in age-appropriate adults, this process has decreased colorectal cancer incidence and saved lives. Screening effectively detects early symptoms, allowing patients, families, and providers to initiate early treatment.
Theoretical Implications
The project confirmed that using the SATIS-PHI/CRC toolkit to redesign the screening process helped improve the screening rates and the early detection of CRC. Furthermore, given the rigor with which the project was implemented, the data analysis procedures reinforce the credibility of these results. For example, the adopted quasi-experimental design is a valid and reliable approach to establishing cause-and-effect associations among different variables.
While this project is robust and its conclusions are credible, it is essential to acknowledge that it suffers shortcomings that may undermine its validity and reliability. For example, the small sample raises questions about the generalizability of the findings. It could be that the observations made are only particular to the 100 participants who took part in the project. Another limitation of the project lies in the absence of controls. Measures for control for extraneous variables were not taken. Therefore, there is a risk that the results obtained could result from some other factor instead of the implemented intervention. While these flaws and drawbacks are significant, they do not necessarily distract from the project’s quality. As will be shown below, this project provides important insights that can serve as the foundation for further research and inform healthcare practice.
The project has confirmed the validity of the theoretical framework upon which it was established. The health belief model and the protection motivation theory are the primary models that comprise the framework. The health belief model holds that individuals are more likely to adopt preventive measures when they feel that the threat they face is severe and has a high likelihood of occurring, and when they believe that the preventive intervention is effective and addresses perceived barriers. CRC screening is effective. After all, it relates to an illness that primarily afflicts adults aged between 50 and 75 and alleviates the cost barrier because it is a cost-effective solution. By establishing the effectiveness of the AHRQ’s SATIS-PHI/CRC toolkit, the project essentially validated the health belief model.
Similarly, the project demonstrated that the protection motivation theory is valid. The basic premise of this theory is that self-efficacy and vulnerability to a threat are factors that influence health behaviors. The 100 participants took the step to undergo screening because they understood that they were particularly vulnerable to CRC and could take simple actions to reduce their exposure. Thus, in closing, the project showed that the two theories are robust tools that can be incorporated into health promotion and disease prevention programs.
Practical Implications
The main practical implication that this project has generated concerns how to reduce CRC cases through effective screening. CRC remains highly prevalent in the U.S. and, measures put in place do not seem effective. This project has clarified that screening is a simple solution that healthcare providers can adopt to protect their patients against CRC. Furthermore, the project highlighted how such instruments as the System Approach to Tracking and Increasing Screening for Public Health Improvement of Colorectal Cancer toolkit can facilitate the design of personalized screening programs that empower patients to become involved in the CRC prevention process.
Additionally, it is evident that for screening to be effective, it should be accompanied by training. Therefore, healthcare institutions should train their personnel on using the System Approach to Tracking and Increasing Screening for Public Health Improvement of Colorectal Cancer toolkit and other items that comprise the CRC prevention measures that they put in place. Perhaps the most important practical implication emerging from the project is that practitioners and providers should give special attention to the detection and treatment of early-stage CRC. Through screening, they can identify patients who need surveillance and patients with symptoms of early-stage CRC and provide them with the necessary treatment to ensure that their condition does not worsen.
Future Implications
In addition to the issues addressed above, the project also has implications for the future. First, since the project established that the SATIS-PHI/CRC toolkit works in redesigning the screening process, it could inspire hospitals and other healthcare facilities to abandon traditional techniques that they have historically used without the desired outcome. If the results of this project are incorporated into practice, CRC screening for the most vulnerable adults will likely become routine. Secondly, the project has left some gaps that could motivate scholars to undertake future research. For example, the project failed to determine whether racial minority status is among the risk factors for CRC. A call is now issued for researchers to consider examining race’s impact on the effectiveness of CRC screening efforts and a redesign of the screening process.
Recommendations
Identifying at-risk individuals for colorectal cancer and providing necessary education that encourages and facilitates early screening is essential. Screening serves as a preventive mechanism that can minimize health disparities, reduce healthcare burden and maximize the quality of life for age-appropriate individuals. Therefore, early identification of disease processes allows for the introduction of appropriate screening/ surveillance approaches, leading to earlier cancer diagnosis and underpinning improved survival (Lawler et al., 2018).
Recommendations for Future Projects
This project offers many lessons for future undertakings. First, the project has underscored the need for a large sample. Therefore, the PI will recruit a larger and more representative sample of the broader population for future projects. This recommendation is based on the fact that the sample of 100 patients is too small to generate conclusions that can be generalized. Secondly, the PI shall work with a bigger team for future projects. During the implementation of the project, it was difficult for the team to complete all tasks. This problem threatened the project and could be used to raise questions about the credibility of the entire project.
Therefore, to ensure that future projects are undertaken smoothly, the PI intends to work with more professionals. Thirdly, future projects will be conducted in a setting more representative of the general population’s conditions. While New York is racially and socioeconomically diverse, it does not necessarily reflect the situation in the rest of the country. Therefore, future projects will be undertaken in an environment that allows generalizable insights to be obtained. Lastly, another recommendation that will guide the implementation of future projects is variable controls. For the current project, controls for extraneous variables were not included. As a result, there is some chance that the results of the project lack robustness.
While the project is comprehensive and credible, some gaps should be addressed as part of further projects. For example, the project did not draw clear conclusions regarding race’s role in preventing CRC using screening. These questions should be addressed in future projects.
Recommendations for Practice
Several recommendations are a result of healthcare practice. First, it is advised that medical service providers adopt the AHRQ’s SATIS-PHI/CRC toolkit as part of their programs to increase CRC screening and prevent CRC in some cases. While they do not necessarily need to use the System Approach to Tracking and Increasing Screening for Public Health Improvement of Colorectal Cancer toolkit, the providers are welcome to establish their screening protocols on this instrument. Secondly, it is proposed that medical facilities undertake rigorous training to prepare their practitioners to implement the toolkit. These facilities should also ensure that the screening that they conduct creates room for patients to participate. Patients and healthcare practitioners are among the key stakeholders who stand to benefit from reading this project report.
On the one hand, the report provides practical steps such as screening that patients can take to reduce their risk of developing CRC. On the other hand, the project confirmed that AHRQ’s SATIS-PHI/CRC toolkit effectively increases CRC screening rates at the facility used for the project. Because the tool proved to be effective in improving the screening rate of CRC, practitioners should include it in their CRC prevention initiatives.
References
Abotaleb, A. (2018). Cost of illness for colorectal cancer at low middle-income countries Egypt case.Annals of Oncology, 29(5), v45-v55. Web.
Abualkhair, W. H., Zhou, M., Ahnen, D., Yu, Q., Wu, X., & Karlitz, J. J. (2020). Trends in incidence of early-onset colorectal cancer in the United States among those approaching screening age. JAMA Network Open, 3(1), e1920407. doi: 10.1001/jamanetworkopen. 2019.20407
Agency for Healthcare Research and Quality. (2018a). Tracking and improving screening for colorectal cancer intervention. Background. Web.
Agency for Healthcare Research and Quality (AHRQ). (2018b). Health care systems for tracking colorectal cancer screening tests. Web.
Aiello, P., Sharghi, M., Mansourkhani, S. M., Ardekan, A. P., Jouybari, L., Daraei, N., Peiro, K., Mohamadian, S., Rezaei, M., Heidari, M., Peluso, I., Ghorat, F., Bishayee, A., & Kooti, W. (2019). Medicinal plants in the prevention and treatment of colon cancer. Oxidative Medicine and Cellular Longevity, 2019(10), 1-53. Web.
Alnaif, M. S., & Alghanim, S. A. (2009). Patients’ knowledge and attitudes towards health education: implications for primary health care services in Saudi Arabia. Journal of Family & Community Medicine, 16(1), 27–32.
Altman, M. R., Colorafi, K., & Daratha, K. B. (2018). The reliability of electronic health record data used for obstetrical research. Applied Clinical Informatics, 9(1), 156–162. Web.
Alyabsi, M., Meza, J., Islam, K. M. M., Soliman, M., & Watanabe-Galloway, S. (2020). Colorectal cancer screening uptake: Differences between rural and urban privately-insured population. Frontiers in Public Health, 8(532950), 1-10. Web.
American Cancer Society. (2014). Colorectal cancer facts & figures 2014-2016. Web.
American Cancer Society. (2017). Colorectal cancer facts & figures 2017-2019. Atlanta: American Cancer Society; 2017.
American Cancer Society. (2020). Colorectal cancer facts & figures 2020-2022. Atlanta: American Cancer Society; 2020.
American Cancer Society. (2021a). Key statistics for colorectal cancer. Web.
American Cancer Society. (2021b). Colorectal cancer ACS research highlights. Web.
Amitay, E. L., Carr, P. R., Jansen, L., Roth, W., Alwers, E., Herpel, E., Kloor, M., Blaker, H., Chang-Claude, J., Brenner, H., & Hoffmeister, M. (2020). Smoking, alcohol consumption and colorectal cancer risk by molecular pathological subtypes and pathways. British Journal of Cancer, 122(11), 1604-10.
Aqtam, I., & Darawwad, M. (2018). Health promotion model: An integrative literature review. Open Journal of Nursing, 8(7), 485-503.
Arnold, C. L., Rademaker, A. W., Morris, J. D., Ferguson, L. A., Wiltz, G., & Davis, T. C. (2019). Follow‐up approaches to a health literacy intervention to increase colorectal cancer screening in rural community clinics: A randomized controlled trial. Cancer, 125(20), 3615-22.
Arnold, M., Sierra, M. S., Laversanne, M., Soerjomataram, I., Jemal, A., & Bray, F. (2017). Global patterns and trends in colorectal cancer incidence and mortality. Gut, 66(4), 683-91.
Bhandari, P. (2021). Type I and type II errors. Web.
Binu, V. S., Mayya, S. S., & Dhar, M. (2014). Some basic aspects of statistical methods and sample size determination in health science research. Ayu, 35(2), 119–123. Web.
Blum-Barnett, E., Madrid, S., Burnett-Hartman, A., Mueller, S. R., McMullen, C. K., Dwyer, A., & Feigelson, H. S. (2019). Financial burden and quality of life among early‐onset colorectal cancer survivors: A qualitative analysis. Health Expectations, 22(5), 1050-7.
Bone, R. H., Cross, J. D., Dwyer, A. J., Fox, J. L., Hyams, D. M., Hassmiller, L. K., Mackey, T. A., Miller, R. M., & Fendrick, A. M. (2020). A path to improve colorectal cancer screening outcomes: Faculty roundtable evaluation of cost-effectiveness and utility. American Journal of Managed Care, 26(6 Suppl), S123-43.
Bornstein, S. (2012). An integrated EHR at Northern California Kaiser Permanente: pitfalls, challenges, and benefits experienced in transitioning.Applied Clinical Informatics, 3(3), 318–325. Web.
Boscoe, F. P., Johnson, C. J., Sherman, R. L., Stinchcomb, D. G., Lin, G., & Henry, K. A. (2014). The relationship between area poverty rate and site-specific cancer incidence in the United States. Cancer, 120(14), 2191–2198. Web.
Brenner, H., & Chen, C. (2018). The colorectal cancer epidemic: Challenges and opportunities for primary, secondary and tertiary prevention. British Journal of Cancer, 119(7), 785-92.
Brenner, H., Jansen, L., Ulrich, A., Chang-Claude, J., & Hoffmeister, M. (2016). Survival of patients with symptom- and screening-detected colorectal cancer. Oncotarget, 7(28), 44695–44704.
Brysbaert, M. (2019). How many participants do we have to include in properly powered experiments? A tutorial of power analysis with reference tables. Journal of Cognition, 2(1), 16. Web.
Canadian Task Force on Preventive health Care. (2016). Canadian Medical Association Journal publishes CTFPHC’s guideline on colorectal cancer screening in adults. Web.
Cancino, R. S., Su, Z., Mesa, R., Tomlinson, G. E., & Wang, J. (2020). The impact of COVID-19 on cancer screening: Challenges and opportunities. JMIR Cancer, 6(2), e21697.
Carmichael, H., Cowan, M., McIntyre, R., & Velopulos, C. (2020). Disparities in colorectal cancer mortality for rural populations in the United States: Does screening matter? American Journal of Surgery, 219(6), 988-92.
Centers for Disease Control and Prevention (CDC). (2012). Principles of epidemiology in public health practice, 3rd. ed, lesson 3: An introduction to applied epidemiology and biostatistics. Web.
Centers for Disease Control and Prevention (CDC). (2013). Colorectal cancer screening rates remain low. Web.
Centers for Disease Control and Prevention (CDC). (2020). Colorectal cancer statistics. Web.
Centers for Disease Control and Prevention. (2021a). What is colorectal cancer? Web.
Centers for Disease Control and Prevention (CDC). (2021b). What should I know about screening? Web.
Centers for Medicare & Medicaid Services. (2021). Healthcare Effectiveness Data and Information set (HEDIS). Web.
Chiu, A. (2020). Chadwick Boseman’s death is making young people think about colon cancer. Here’s what to know. The Washington Post. Web.
Cohen, J. (1988). Statistical power analysis for the behavioral sciences. Routledge Academic
Coughlin, S. S. (2020). Social determinants of colorectal cancer risk, stage, and survival: A systematic review. International Journal of Colorectal Diseases. 2020;35(6):985-995. Web.
Creswell, J., & Poth, C. (2017). Qualitative inquiry and research design: Choosing among five approaches. Sage, London.
Daniel, E. (2016). The usefulness of qualitative and quantitative approaches and methods in researching problem-solving ability in science education curriculum. Journal of Education and Practice, 7(15), 91-100.
DeGroff, A., Sharma, K., Satsangi, A., Kenney, K., Joseph, D., Ross, K., Leadbetter, S., Helsel, W., Kammerer, W., Firth, R., Rockwell, T., Short, W., Tangka, F., Wong, F., & Richardson, L. (2018). Increasing colorectal cancer screening in health care systems using evidence-based interventions.Preventing Chronic Disease, 15(E100), 1-15. Web.
Devault, G. (2019). Advantages and disadvantages of quantitative research. Web.
Doubeni, C. (2021).Screening for colorectal cancer: Strategies in patients at average risk. Web.
Dowd, M. (2018). Advantages and disadvantages of qualitative and quantitative research. Web.
Eaglehouse, V. L., Georg, M. W., Richard, P., Shriver, C. D., & Zhu, K. (2019). Costs for colon cancer treatment comparing benefit types and care sources in the US military health system. Military Medicine, 184(11-12), e847-55.
Ebell, M., Shaughnessy, A., & Slawson, D. (2018). Why are we so slow to adopt some evidence-based practices? American Family Physician, 98(12), 709-710.
Gaille, L. (2019).13 Pros and cons of quantitative research methods. Vittana. Web.
Gong, R., & Xie, M. (2019). Discussion on prior-based Bayesian information criterion (PBIC). In: M. J. Bayarri, James O. B., Woncheol J., Surajit R., Luis R., Pericchi, &Ingmar, V. Statistical Theory and Related Fields, 3(1), 35-36. Web.
Gonzalez, S. J., Mejia de Grubb, M. C., & Levine, R. S. (2017). Primary and secondary prevention of colorectal cancer: An evidence-based review. Family Medicine and Community Health, 5(1), 75-84.
Hair, Jr., J. F., Anderson, R. E., Tatham, R. L., & Black, W. C. (1998) Multivariate data analysis. Fifth Edition. Prentice-Hall:New Jersey.
Harris, D. M., & Borsky, A. E. (2010). Health care systems for tracking colorectal cancer screening tests: Final report on the system approach to tracking and increasing Screening for Public Health Improvement of Colorectal Cancer (S A T I S-PHI/CRC) intervention. AHRQ. Web.
Henry, K. A., Sherman, R. L., McDonald, K., Johnson, C. J., Lin, G., Stroup, A. M., & Boscoe, F. P. (2014). Associations of census-tract poverty with subsite-specific colorectal cancer incidence rates and stage of disease at diagnosis in the United States. Journal of Cancer Epidemiology, 2014, 1-12.
Hochbaum, G., Rosenstock, I., & Kegels, S. (1952). Health belief model. United States Public Health Service.
Honein-AbouHaidar, G. N., Kastner, M., & Vuong, V. (2016). Systematic review and meta-study synthesis of qualitative studies evaluating facilitators and barriers to participation in colorectal cancer screening. Cancer Epidemiol. Biomark. Prevention, 25(6), 907–917.
Horshauge, P. M., Gabel, P., Larsen, M. B., Kirkegaard, P., Edwards, A., & Andersen, B. (2020). The association between health literacy and colorectal cancer screening uptake in a publicly funded screening program in Denmark: Cross-sectional study. Preventive Medicine Reports, 19, 101132. Web.
Huang, J. J., & Huang, J. L. W. (2017). Challenges to the adoption of risk algorithms for colorectal cancer screening programs: Perspectives for future research. Hong Kong Medical Journal, 23(6), 661-3.
Huang, X., Yang, Z., Xie, Q., Zhang, Z., Zhang, H., & Ma, J. (2019). Natural products for treating colorectal cancer: A mechanistic review. Biomedicine & Pharmacotherapy, 117 (109142), 1-13. Web.
Hull, M. A., Rees, C. J., Sharp, L., & Koo, S. (2020). A risk-stratified approach to colorectal cancer prevention and diagnosis. Nature Reviews Gastroenterology & Hepatology, 17, 773-80. Web.
Jackson, C. S., Oman, M., Patel, A. M., & Vega, K. J. (2016). Health disparities in colorectal cancer among racial and ethnic minorities in the United States. Journal of Gastrointestinal Oncology, 7(Suppl 1), S32-43.
Jaklevic, M. C. (2021). Pandemic spotlights in-home colon cancer screening tests. JAMA, 325(2), 116–118. Web.
Johnson, R. J. (2016). A comprehensive review of an electronic health record system soon to assume market ascendancy: EPIC®. Journal of Healthcare Communications, 1(4), 1-4. DOI: 10.4172/2472-1654.100036
Jones, C. L., Jensen, J. D., Scherr, C. L., Brown, N. R., Christy, K., & Weaver, J. (2015). The health belief model as an explanatory framework in communication research: Exploring parallel, serial, and moderated mediation. Health Communication, 30(6), 566–576. Web.
Kent State University. (2021). SPSS tutorials: Paired samples t test. Web.
Keum, N., & Giovannucci, E. (2019). Global burden of colorectal cancer: Emerging trends, risk factors and prevention strategies. Nature Reviews Gastroenterology & Hepatology, 16, 713-32.
Khalili, F., Najafi, B., Mansour-Ghanaei, F., Yousefi, M., Abdollahzad, H., & Motlagh, A. (2020). Cost-effectiveness analysis of colorectal cancer screening: A systematic review. Risk Management and Healthcare Policy, 13, 1499–1512.Web.
Khodaveisi, M., Omidi, A., Farokhi, S., & Soltanian, A. R. (2017). The effect of Pender’s health promotion model in improving the nutritional behavior of overweight and obese women. International Journal of Community Based Nursing and Midwifery, 5(2), 165–174
Kim, Y., & Steiner, P. (2016). Quasi-experimental designs for causal inference. Journal of Educational Psychology, 51(3-4), 395-405.
Knake, L. A., Ahuja, M., McDonald, E. L., Ryckman, K. K., Weathers, N., Burstain, T., Dagle, J. M., Murray, J. C., & Nadkami, P. (2016). Quality of EHR data extractions for studies of preterm birth in a tertiary care center: guidelines for obtaining reliable data. BMC Pediatrics, 16(59), 1-8. Web.
Kollman, J., & Sobotka, H. L. (2018). Poverty and cancer disparities in Ohio. Preventing Chronic Disease, 15(E152), 1-10. Web.
Lawler, M., Alsina, D., Adams, R. A., Anderson, A. S., Brown, G., Fearnhead, N. S., Fenwick, S. W., Halloran, S. P., Hochhauser, D., Hull, M. A., Koelzer, V. H., McNair, A., Monahan, K. J., Näthke, I., Norton, C., Novelli, M. R., Steele, R., Thomas, A. L., Wilde, L. M., … Tomlinson, I. (2018). Critical research gaps and recommendations to inform research prioritization for more effective prevention and improved outcomes in colorectal cancer. Gut, 67(1), 179–193. Web.
Levin, T. R., Corley, D. A., Jensen, C. D., Schottinger, J. E., Quinn, V. P.,Zauber, A. G., Lee, J. K., Zhao, W. K., Udaltsova, N., Ghai, N. R., Lee, A. T., Quesenberry, C. P., Fireman, B. H., & Doubeni, C. A. (2018). Effects of organized colorectal cancer screening on cancer incidence and mortality in a large community-based population. Gastroenterology, 155(5), 1383–1391. Web.
Lied, T. R., Malsbary, R., Eisenberg, C., & Ranck, J. (2002). Combining HEDIS indicators: a new approach to measuring plan performance. Health Care Financing Review, 23(4), 117–129.
Loke, A. Y., Davies, L., & Li, S. (2015). Factors influencing the decision that women make on their mode of delivery: The health belief model.BMC Health Services Research, 15(274). Web.
Lor, M., Backonja, L., & Lauver, D. R. (2017). How could nurse researchers apply theory to generate knowledge more efficiently? Journal of Nursing Scholarship, 49(5), 580-9.
Maciejewski, K. M. (2020). Quasi-experimental design. Biostatistics & Epidemiology, 4(1), 38-47.
Mantwill, S., Monestel-Umaña, S., & Schulz, P. J. (2015). The relationship between health literacy and health disparities: A systematic review. PLoS ONE, 10(12), e0145455. Web.
Mariotto, A. B., Warren, J. L., Zeruto, C., Coughlan, D., Barrett, M. J., Zhao, L., & Yabroff, K. R. (2020). Cancer-attributable medical costs for colorectal cancer patients by phases of care: What is the effect of a prior cancer history? Monographs, 55, 22-30.
McDonald, M., & Shenkman, L. J. (2018). Health literacy and health outcomes of adults in the United States: Implications for providers. The Internet Journal of Applied Health Sciences and Practice, 16(4), article 2.
Medicare Advantage Plans. (2021). 2021 HEDIS reference guide for primary care: Colorectal cancer screening (COL). Web.
Miller, D. P., Brownlee, C. D., McCoy, T. P., & Pignone, M. P. (2007). The effect of health literacy on knowledge and receipt of colorectal cancer screening: a survey study.Biomedical Central Family Practice, 8(16), 1-7. Web.
Miller, K. (2017). “Doing something exceptional”: Rural communities and colorectal cancer screening. Rural Health Info. Web.
Modica, C., Lewis, J. H., & Bay, C. (2019). Colorectal cancer: Applying the value transformation framework to increase the percent of patients receiving screening in federally qualified health centers.Preventive Medicine Reports, 15, 1-7. Web.
Montgomery, E. B. (2021). The patient as parenthetical. In: The ethics of everyday medicine, Academic Press.
Montminy, E. M., Karlitz, J. J., & Landreneau, S. W. (2019). Progress of colorectal cancer screening in United States: Past achievements and future challenges. Preventive Medicine, 120(2019), 78-84.
Mrabti, H., Amziren, M., ElGhissassi, I., Bensouda, Y., Berrada, N., Abahssain, H., Boutayeb, S., El Fakir, S., Nejjari, C., Benider, A., Mellas, N., El Mehsabi, O., Bennani, M., Bekkali, R., Zidouh, A., & Errihani, H. (2016). Quality of life of early stage colorectal cancer patients in Morocco.BMC Gastroenterology, 16(2016), 131-41. Web.
National Committee for Quality Assurance (NCQA). (2016). Technical specifications update. Web.
National Committee for Quality Assurance (NCQA). (2017). NCQA releases 2017 CRC HEDIS measure. Web.
National Committee for Quality Assurance. (2021). HEDIS measures and technical resources. Web.
New York State Cancer Registry. (2021). Colon cancer incidence and mortality by age group, Bronx County, 2014-2018. Web.
O’Connor, J. M., Sedghi, T., Dhodapkar, M., Kane, M. J., & Gross, C. P. (2018). Factors associated with cancer disparities among low-, medium-, and high-income US counties. JAMA Network Open, 1(6), e183146. doi:10.1001/jamanetworkopen.2018.3146
Paita, L., Love, D., Lertwachara, K., & Grabovsky, I. (2003). Evaluating the reliability and validity of the Health Plan Employee Data and Information Set. Web.
Patel, S., Issaka, R. B., Chen, E., & Somsouk, M. (2021). Colorectal cancer screening and COVID-19. The American Journal of Gastroenterology, 116(2), 433–434. Web.
Polit, D. F., & Beck, C. T. (2017). Nursing research: Generating and assessing evidence for nursing practice (10th Ed.). Wolters Kluwer.
Rakhshanderou, S., Maghsoudloo, M., Safari-Moradabadi, A., & Ghaffari, M. (2020). Theoretically designed interventions for colorectal cancer prevention: a case of the health belief model. BMC Medical Education, 20(2020), 270-8. Web.
Ran, T., Cheng, C. Y., Misselwitz, B., Brenner, H., Ubels, J., & Schlander, M. (2019). Cost-effectiveness of colorectal cancer screening strategies-A systematic review. Clinical Gastroenterol Hepatology. 2019 Sep;17(10):1969-1981.e15. Web.
Ransohoff, D. F., & Sox, H. C. (2016). Clinical practice guidelines for colorectal cancer screening. New recommendations and new challenges. JAMA, 315(23), 2529-31.
Ratjen, I., Schafmayer, C., Enderle, J., Di Giuseppe, R., Waniek, S., Koch, M., Burmeister, G., Nothlings, U., Hampe, J., Schlesinger, S., & Lieb, W. (2018). Health-related quality of life in long-term survivors of colorectal cancer and its association with all-cause mortality: a German cohort study. BMC Cancer, 18(1), 1156. Web.
Rawla, P., Sunkara, T., & Barsouk, A. (2019). Epidemiology of colorectal cancer: incidence, mortality, survival, and risk factors. Przegla̜d Gastroenterologiczny, 14(2), 89-103.
Reeves, S., Pelone, F., Harrison, R., Goldman, J., & Zwarenstein, M. (2017). Interprofessional collaboration to improve professional practice and healthcare outcomes. Cochrane Database of Systematic Reviews, 6(6), CD000072. Web.
Rock, C. L., Thomson, C., Gansler, T., Gapstur, S. M., McCullough, M. L., Patel, A. V., Andrews, K. S., Bandera, E. V., Spees, C. K., Robien, K., Hartman, S., Sullivan, K., Grant, B. G., Hamilton, K. K., Kushi, L. H., Caan, B. J., Kibbe, D., Black, J. D., Wiedt, T. L., … Doyle, C. (2020). American Cancer Society guideline for diet and physical activity for cancer prevention. CA: A Cancer Journal for Clinicians, 70(4), 245-71.
Rogers, C. R., Blackburn, B. E., Huntington, M., Curtin, K., Thorpe, R. J., Rowe, K., Snyder, J., Deshmukh, V., Newman, M., Fraser, A., Smith, K., & Hashibe, M. (2020). Rural-urban disparities in colorectal cancer survival and risk among men in Utah: a statewide population-based study. Cancer Causes & Control, 31(3), 241-53.
Rogers, R. W. (1975). A protection motivation theory of fear appeals and attitude change. Journal of Psychology, 91(1), 93-114.
Rosenstock, I. M. (1974). The health belief model and preventive health behavior. Health Education Monographs, 2(4), 354-86.
Ross, P. T., & Bibler Zaidi, N. L. (2019). Limited by our limitations. Perspectives on Medical Education, 8(4), 261–264. Web.
Sarfaty, M., Myers, R. E., Harris, D. M., Borsky, A. E., Sifri, R., Cocroft, J., Stello, R., & Johnson, M. (2012). Variation in colorectal cancer screening steps in primary care: Basis for practice improvement. American Journal of Medical Quality, 27(6), 458-66.
Schleimann, D., Matovu, M., Ramanathan, K., Munoz-Aguirre, P., O’Neill, C., Kee, F., Su, T. T., & Donnelly, M. (2020). Implementation of colorectal cancer screening interventions in low-income and middle-income countries: a scoping review protocol.BMJ Open, 10(6), e037520. Web.
Schweizer, M. L., Braun, B. I., & Milstone, A. M. (2016). Research Methods in Healthcare Epidemiology and Antimicrobial Stewardship-Quasi-Experimental Designs. Infection Control and Hospital Epidemiology, 37(10), 1135–1140. Web.
Seematter-Bagnoud, L., & Büla, C. (2018). Brief assessments and screening for geriatric conditions in older primary care patients: a pragmatic approach.Public Health Reviews, 39(8), 1-13. Web.
Siddiqui, T. R., Ghazal, S., Bibi, S., Ahmed, W., & Sajjad, S. F. (2016). Use of the health belief model for the assessment of public knowledge and household preventive practices in Karachi, Pakistan, a dengue-endemic city. Plos Neglected Tropical Diseases, 10(11), e0005129. Web.
Siegel, R. L., Miller, K. D., Goding Sauer, A., Fedewa, S. A., Butterly, L. F., Anderson, J. C., Cercek, A., Smith, R. A., & Jemal, A. (2020). Colorectal cancer statistics. CA: A Cancer Journal for Clinicians,70(3), 145-164.
Sierra, M. S., & Forman, D. (2016). Burden of colorectal cancer in Central and South America. Cancer Epidemiology, 44(Suppl 1), S74-81.
Sims, J. M. (2010). A brief review of the Belmont report. Dimensions of Critical Care Nursing, 29(4), 173-4.
Singh, S. G., & Aiken, J. (2017). The effect of health literacy level on health outcomes in patients with diabetes at a type v health centre in Western Jamaica. International Journal of Nursing Sciences, 4(3), 266-70.
Smith, R. A., Andrews, K. S., Brooks, D., Fedewa, S. A., Manassaram-Baptiste, D., Saslow, D., & Wender, R. C. (2019). Cancer screening in the United States, 2019: A review of current American Cancer Society guidelines and current issues in cancer screening. CA: A Cancer Journal for Clinicians, 69(3), 184-210.
Solmi, F., Von Wagner, C., Kobayashi, L. C., Raine, R., Wardle, J., & Morris, S. (2015). Decomposing socio-economic inequality in colorectal cancer screening uptake in England. Social Science & Medicine. 134 (2015), 76-86. Web.
Steidl, M. S., & Zimmern, P. (2013). Data for free–can an electronic medical record provide outcome data for incontinence/prolapse repair procedures? Journal of Urology, 189(1):194-9. Web.
Stormacq, C., Van den Broucke, S., & Wosinski, J. (2019). Does health literacy mediate the relationship between socioeconomic status and health disparities? Integrative review. Health Promotion International, 34(5), e1-e17. Web.
Subramanian, S., Tangka, F., Hoover, S., Royalty, J., DeGroff, A., & Joseph, D. (2017). Costs of colorectal cancer screening provision in CDC’s Colorectal Cancer Control Program: Comparisons of colonoscopy and FOBT/FIT based screening. Evaluation and Program Planning, 62, 73–80. Web.
Sullivan, G. M., & Feinn, R. (2012). Using Effect Size-or Why the P Value Is Not Enough. Journal of Graduate Medical Education, 4(3), 279–282. Web.
Swaminathan, R., Morris, J. D., & Davis, T. C. (2020). Rural-urban disparities in colorectal cancer screening: An insight from a statewide database. Journal of Clinical Oncology, 38(4), 69.
Sylvia, M. L., & Terhaar, M. F. (2014). Clinical analytics and data management for the DNP. New York, NY: Springer.
Syriopoulou, E., Morris, E., Finan, P. J., Lambert, P. C., & Rutherford, M. J. (2019). Understanding the impact of socioeconomic differences in colorectal cancer survival: potential gain in life-years. British Journal of Cancer, 120, 1052-8.
Taherdoost, H. (2020). Sampling methods in research methodology: How to choose a sampling technique for research. International Journal of Academic Research in Management, 5(2), 18-27.
Tanaka, A., Zhou, Y., Ogawa, M., Shia, J., Klimstra, D. S., Wang, H. Y., & Roehrl, M. H. (2020). STAT1 as a potential prognosis marker for poor outcomes of early stage colorectal cancer with microsatellite instability. Plos One. 15(4), e0229252. Web.
Taylor, J. (2019). Racism, inequality, and health care for African Americans. The Century Foundation. Web.
Theofanidis, Dimitrios, & Fountouki, Antigoni. (2019). Limitations and delimitations in the research process. Perioperative Nursing (GORNA), E-ISSN:2241-3634, 7(3), 155–162. Web.
Thomas, L. (2020). An introduction to quasi-experimental designs. Web.
US Preventive Services Task Force. (2016). Screening for colorectal cancer: US Preventive Services Task Force: Recommendation statement. JAMA. 315(23):2564–2575. 10.1001/jama.2016.5989
US Preventive Services Task Force. (2021). Screening for colorectal cancer. JAMA. 325(19):1965-1977. doi:10.1001/jama.2021.6238.
Wangmar, J., Jervaeus, A., Fritzell, K., Wångdahl, J., Hultcrantz, R., & Wengström, Y. (2018). Health literacy levels and views about being invited to a colorectal cancer screening program. Acta Oncologica, 57(6), 43-749
Westcott, R., Ronan, K., Bambrick, H., & Taylor, M. (2017). Expanding protection motivation theory: investigating an application to animal owners and emergency responders in bushfire emergencies. BMC Psychology, 5(1), 13. Web.
White, A., Thompson, T. D., White, M. C., Sabatino, S. A., De Moor, J., Doria-Rose, P. V., Geiger, A. M., & Richardson, L. C. (2017). Cancer screening test use — United States, 2015. Morbidity and Mortality Weekly Report, 66(8), 201-206.
White, H., & Sabarwal, S. (2014). Quasi-experimental design. UNICEF. Web.
White, M. C., Kavanaugh-Lynch, M. M., Davis-Patterson, S., & Buermeyer, N. (2019). An expanded agenda for the primary prevention of breast cancer: Charting a course for the future. International Journal of Environmental Research and Public Health. 17(714), 1-11.
White, M. P., & Itzkowitz, S. H. (2020). Barriers driving racial disparities in colorectal cancer screening in African Americans.Current Gastroenterology Reports, 22(8), 41. Web.
Wilkins, T., McMechan, D., & Talukder, A. (2018). Colorectal cancer screening and prevention. American Family Physician, 15(97), 658-65.
Wise, J. (2016). Childhood poverty is linked to adult cancer risk, says report. BMJ 2016(353), i2808. Web.
Woudstra, A. J., Smets, E., Verdam, M., & Fransen, M. P. (2019). The role of health literacy in explaining the relation between educational level and decision making about colorectal cancer screening. International Journal of Environmental Research and Public Health, 16(23), 4644. Web.
Yip, C., Han, N. R., & Sng, B. L. (2016). Legal and ethical issues in research.Indian Journal of Anaesthesia, 60(9), 684–688. Web.
Yuan, Y., Yu, K., Shen, Y., Dickinson, J., & Winget, M. (2018). Importance of quality in breast cancer screening practice – a natural experiment in Alberta, Canada. BMJ Open, 10(1). Web.
Zahnd, W. E., Gomez, S. L., Steck, S. E., Brown, M. J., Ganai, S., Zhang, J., Adams, S. A., Berger, F. G., & Eberth, J. M. (2021). Rural‐urban and racial/ethnic trends and disparities in early‐onset and average‐onset colorectal cancer. Cancer, 127(2), 239-48.
Zapka, J. (2008). Innovative provider and health system-directed approaches to improving colorectal cancer screening delivery. Medical Care, 46(9), S62-S67.
Zare, M., Ghodsbin, F., Jahanbin, I., Ariafar, A., Keshavarzi, S., & Izadi, T. (2016). The effect of health belief model-based education on knowledge and prostate cancer screening behaviors: A randomized controlled trial. International Journal of Community Based Nursing and Midwifery, 4(1):57-68.