Introduction
Conductions remain to be the main hindrance to improvement in lithium-ion (also referred to as Li-ion) batteries. Despite the technological advancements in the recent world, this barrier is seen to remain for foreseen future. A comprehensive survey on the conduction phenomena in Li-ion cells is done to gain a good understanding of the process and to enable a breakthrough in technology (Azevedo, Baldan, and Ferreira 2).
A good example of a family of rechargeable batteries is the lithium-ion (also referred to as Li-ion battery). Lithium ions move from the negative electrode to the positive electrode when discharging. During discharging, the lithium ions move from the positive electrode to the negative electrode. The safety characteristics, performance, and chemistry vary in the different types of LI-ion. Li-ion electrochemical cells apply lithium compound as the electrode material in place of metallic lithium.
Enhanced electronic conductivities coupled with ionic diffusivities in anode and cathode substances or materials are making it possible for improvements in the capacity of lithium batteries. The cathode in this family of batteries is made of metallic oxides, which have low electronic conductivity. The electrolyte is tuned to accept ions while at the same time forming a safe and impenetrable insulating barrier (Azevedo, Baldan, and Ferreira 4).
Researchers in this field face various challenges. These challenges include the low electrical and ionic electronic conductivity values in the electrochemical cells and the unclear relationship between electrical conduction and ionic conductivity in cathode materials. Other challenges the researchers face are the constantly changing conduction properties of the anode and the difficulty in measuring the conductivity and microstructure of the solid-electrolyte film. To design improved materials, the models and materials of battery cells require the best available estimates for diffusivity and conductivity. There is continuous improvement of the knowledge of cell response, which has been contributed by the different models of molecular and atomic, and fibers and continuum scale (Bard and Mirkin 16).
The knowledge about conduction phenomena is still scarce since many researchers do not tackle the problem; instead, they touch narrowly on cathode and anode. A compressive survey on conduction phenomena is done in this paper. Different methods are used to collect data. These methods are simulation, experimental and theoretical studies. The main objectives are to survey electrical and ionic conduction fundamental theories, the relationship between ionic and electrical conduction for Lin2o4, LiMn2O4, and LiFePO4, and review of the phase change in anodes made of graphite (pourbaix 90).
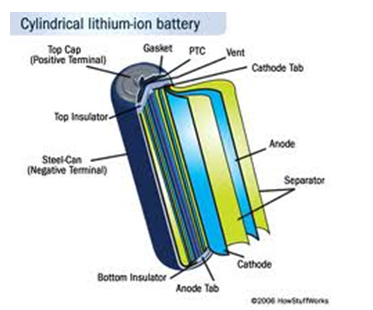
The outer case is made of metal, which pressurizes the cell. It has a vent hole that releases pressure when the battery gets very hot. The Positive Temperature Coefficient (PTC) switch is used to prevent the battery from overheating. The separator separates the positive and the negative electrodes while allowing electrons to move through. Lithium cobalt oxide makes the positive electrode while the negative electrode is made of carbon.
Proposed approaches
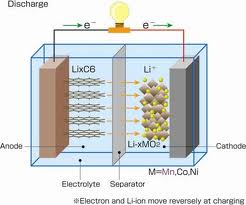
Electrons and ions conduct from cathode to anode in a primary cell and vice versa in a secondary cell. Electrons flow from the cathode to the anode while ions flow from the anode to the cathode. This process involves a redox reaction, whereby the cathode dissipates electrons while the anode accepts the produced electrons. The electrolyte dissociates to ions, both positive and negative. The positive ions (cations) move to the cathode while the negative ions (anions) move to the anode. The strength of the battery depends on the exchange of the oxidation state. The ease of transfer of electrons ions between the cathode and anode can dictate the strength or magnitude of the cell’s potential force. During the discharge of the Li-ion battery, electrons move from anode to cathode (Bard and Mirkin 20).
In addition to electron transfer between the anode and cathode, ionic conduction between the electrolyte and electrodes is vital for the whole process of electrochemical reaction to being complete. Research reveals that the operating voltage of a battery or cell is always lower than the standard voltage of the cell or battery. This is due to potential drops or losses caused by various factors. The standard cell potential, activation polarizations at the cathode and anode, concentration polarization at the cathode and anode, cell operating current, and the internal resistance of the cell are all related to conduction phenomena. Conduction properties of different materials affect the internal resistance (Azevedo, Baldan, and Ferreira 6).
The charge and discharge rate, cycling stability, and practical capacity of a Li-ion battery cell are determined by the diffusion properties of the cell. Fick’s law equation describes the diffusion process. The equation, ![]() , is referred to as Fick’s law, while D is the diffusion coefficient or diffusivity. Diffusion involves the random movement of atoms or ions, which results in an exchange of positions of these particles with their neighbors.
, is referred to as Fick’s law, while D is the diffusion coefficient or diffusivity. Diffusion involves the random movement of atoms or ions, which results in an exchange of positions of these particles with their neighbors.
This process is temperature-dependent. Solids are more temperature-dependent than liquids for diffusion to occur since their particles are firmly held together by strong covalent bonds. The diffusion process in solids can be categorized into two: Defect-mediated mechanisms and non-defect mediated mechanisms. The defect-mediated mechanisms require more energy for activation than the non-defect mediated mechanisms. Similarly, ion diffusion has two primary defects, which affect diffusion in ionic crystals. These include the Schottky pairs, which are the cation vacancy plus the anion vacancy, and Frenkel pairs, which are the cation vacancy plus the cation interstitial (pourbaix 85).
Schottkey defects make ionic solids have lower ionic conductivities and high activation enthalpies. This is because ionic transport occurs from the movement of vacancies. Since ionic transport happens primarily from the movement of interstitial species, crystals with Frenkel defects have high ionic conductivities and low activation enthalpies. Since Li-ions have a very small radius, they diffuse by interstitial mechanism.
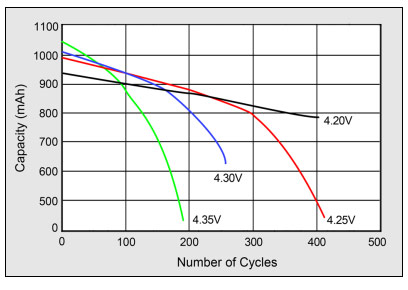
Diffusion in Li-ion is dependent on several factors. As previously pointed out, high temperatures would increase the diffusion rate. In crystals, diffusion is greatly influenced by bonding potential and defects. The strongest bonds known are the ionic and covalent bonds. Ionic bond results when two elements or species transfer electrons from each other while covalent bonding is due to sharing of electrons (Azevedo, Baldan, and Ferreira 10). The diagram below shows the effect on the life cycle at elevated charge voltages.
The ionic and electrical conductivities should be optimized in cathode materials since either of them can dictate the overall cell properties. However, great emphasis is on ionic conductivity rather than ionic conductivity since high ionic conductivity allows rapid diffusion of Li-ions into the cathode. The only successful techniques to increase the conductivity of cathode materials have only been conductive coating and particle size reduction.
There are various schemes for manipulating the diffusivity in cathode particles. These include doping, scaling down the size of the particle, coating the cathode particle surface, and changing the crystallinity. The doping method is widely used by the semiconductor industry to alter silicon conductivity by creating excess charge carriers in the form of positively charged holes and negatively charged electrons. The same concept is used when doping cathode materials (pourbaix 85).
Doping cathodes have not had any improvement in diffusivity of cathodes since the absolute value of the diffusivity still falls within the measured range. The best and most straightforward method for increasing diffusivity is by reducing the cathode particle size to a very small particle. This reduces diffusion length causing a high diffusion rate. Diffusivity can also be tailored by controlling the ratio between crystalline and amorphous phases. However, no schemes have given a breakthrough to ionic conductivity. The main challenge facing experiments designed for purpose of tailoring diffusivity is that the measured values vary widely depending on the setup of the experiment, measurement, simulation technique or method, and electrode fabrication method (Bard and Mirkin 25).
Even though an ideal electrolyte for Li-ion is yet to be developed, Organic electrolytes are used due to their high ionic conductivities. The primary role of an electrolyte is to provide an ionic path between the cathode and the anode. Hence, the electrolyte should enhance ionic conductivity (Azevedo, Baldan, and Ferreira 12).
Expected results
As discussed previously, conduction has been a barrier to further improvements in Li-ion batteries. The various aspects of these batteries, that is, the cathode, anode, and electrolyte, have been discussed in this review. To optimize the electrical and ionic conductivity in the cathode, a doping method is used to improve the electrical conductivity. Surface coating cathode particles with a material, which is conductive, and using scaled-down particles are viable methods to improve electrical conductivity. Recent demonstrations and mathematical treatments show that fibrous architectures are more effective (Azevedo, Baldan, and Ferreira 13).
Modeling is one of the most vital elements of Li-ion cell research. Multi-scale simulations should be used to design strong materials and battery cells since the careful and considerable reduction of order in modeling is necessary for efficiency. This is because these models are implemented to control devices. Coupled models and experiments are important to enable optimization of conduction in various cell components. There are gaps in the reported scientific literature, which must be fixed with new and improved experiments (Azevedo, Baldan, and Ferreira 15).
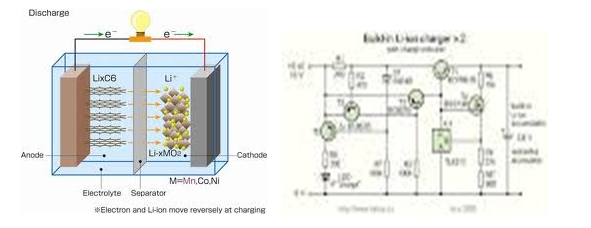
The diagram above shows the movement of ions during charging and discharging. The cathode and anode are doped to increase the electrical conductivity of the battery. The circuit for the implementation of the Li-ion is shown above. Optimization between amorphous and crystalline phases is a very vital strategy for tailoring conductivity in electrodes made of carbon. Efforts have been put in place to replace conventional organic electrolytes. Theoretical and simulation methods are very vital considering electronic and ionic conduction can predict the performance of a battery (Bard and Mirkin 20).

Works Cited
Azevedo, Alves, Malco Baldan, and Neidenei, Ferreira. “Nanodiamond Films for Applications in Electrochemical Systems” International Journal of Electrochemistry 2012.10 (2011):1-16. Print.
Bard, Allen, and Michael, Mirkin. Scanning electrochemical microscopy. New York, Michael Decker, 2001. Print.
Pourbaix, Allard. Atlas of Electrochemical Equilibria in Aqueous Solutions. New York: 1974. Print.