Introduction
The study of acid-base systems is complicated by factors such as the strength of the acid or base being reacted. The strength of an acid or base is a crucial factor because it influences the extent of dissociation hence the overall rate of reaction, which is reflected in the titration curve of the reaction.
For instance, the reaction in a system containing a weak acid or base reveals that the pH at the initial phases of the reaction is high and that the pH changes by a large magnitude before reaching the endpoint. In addition, it is realized that the alterations in pH at the endpoint occur by very small magnitudes. These observations make it extremely difficult to establish the precise endpoint of the reaction particularly if the Ka values of the acid are small.
The Ka value stands for the dissociation constant of an acid, which is the measure of the potency of the acid in the solution. This value is sometimes shown as pKa, which is the logarithm of Ka. A large value of pKa (small Ka value) is characteristic of a weak acid. It shows that an acid dissociates to a small extent. The equivalent weight of an acid can be defined as the mass of an acid that contains one equivalent of hydrogen ions.
The pH of an acid can be computed using the Henderson-Hasselbalch equation, which is written as pH = pKa + log ([A–]/ [HA]). In this equation, pKa is the logarithm of the acid’s Ka value, [A–] is the concentration of the conjugate base in moles, and [HA] is the strength of the acid in moles (before the dissociation). However, the Ka and pKa values of acids can also be found through experiments by measuring the changes in pH of acid upon the addition of small quantities of a base whose concentration is known.
A plot of pH versus volume of base added can then be used to determine the equivalence point of the reaction as well as the pKa of the acid. The pKa is established by the pH of the acid at half the equivalence point. The equivalent weight of acid, on the other hand, can be obtained when the weight of the unknown acid and the equivalence of the base is known.
This experiment aimed at using titration to establish the identity of an unknown acid by finding its molecular weight. The experiment also intended to find the pKa value of the unknown acid.
Procedure
Sodium hydroxide solution was prepared at a concentration of 0.05M by filling a storage bottle with 500 ml of distilled water. Two milliliters of 50% sodium hydroxide solution were then added to the water and mixed thoroughly. A standard potassium hydrogen phthalate solution (KHP) was prepared using the appropriate techniques by weighing 1 g of KHP.
An unknown diprotic acid was then prepared by weighing the amount of the acid as directed by the laboratory instructor and transferring it into a 250 ml volumetric flask. Approximately 200 ml of distilled water was added to the flask followed by agitation to dissolve the entire solid. More distilled water was then added to the volumetric flask up to the mark.
The NaOH titrant solution was then standardized by first transferring 50 ml aliquots of the standard KHP aliquots to three separate 150 ml beakers. Each of the KHP solutions was then titrated against sodium hydroxide. The volume of the sodium hydroxide added against the pH was recorded for each trial. The unknown diprotic acid was also standardized similarly. The equivalence point for each trial was then obtained from the plots of the first or second derivatives.
The concentration of sodium hydroxide used was then computed using the concentration of KHP unknown acid solution and the equivalence points. Thereafter, the average concentration of the NaOH solution and the equivalence points for all the trials of the unknown acid were used to compute the molecular weight of the unknown acid. The pKa value was then obtained from the pH versus volume plot of each of the unknown acid trials.
Results and Data
Table 1: Table of pH versus volume recorded for NaOH and unknown acid.
Calculations
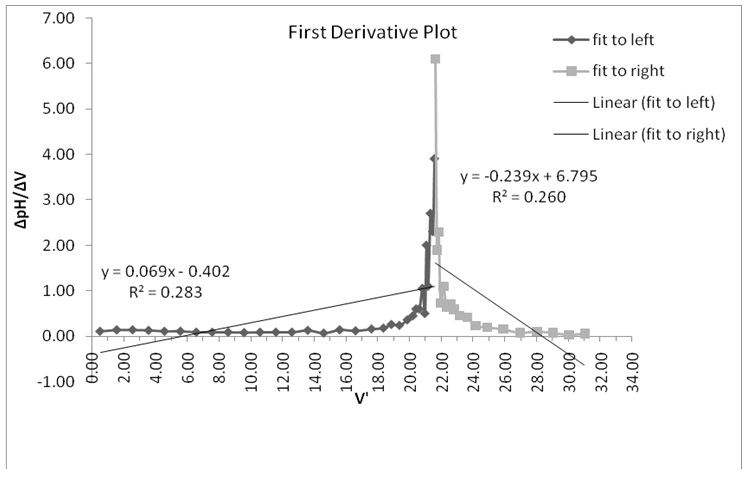
- The equivalence point was determined from the point of intersection in Figure 1 which was equivalent to 21.75 ml.
- The concentration of NaOH used
Sodium hydroxide and potassium hydrogen phthalate reacted according to the chemical equation
NaOH (aq) + KH(C8H4O4) (aq) → NaK(C8H4O4) (aq) + H2O (l).
The concentration of the NaOH solution used was found to be 0.0518 M.
- The equivalent and molecular weight of the unknown acid
If the concentration of NaOH was 0.0518M, then the number of moles contained in 21.75 ml of NaOH was equal to (0.0518×21.75ml)/1000ml= 0.00112665 moles.
Equivalent weight= grams of acid/moles of base
1.007 grams were present in 250 ml of acid, therefore the weight in 50 ml of acid was equal to (1.007g×50 ml)/250 ml=0.2 g
Equivalent weight= 0.2014 g/0.00112665 moles = 178.76 g/mole
(Literature value =204.22g/mole; percentage error= -12.46%).
- The pKa of the unknown acid
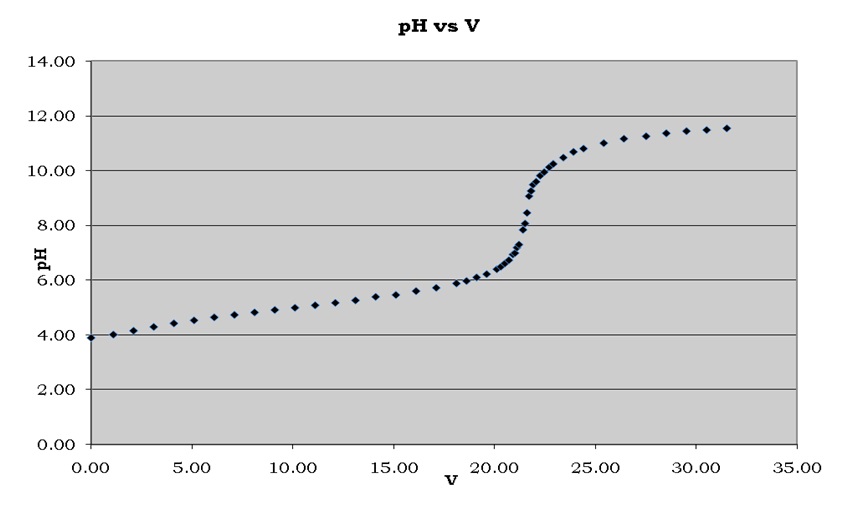
The pKa was given by the pH that coincided with half the volume of the equivalence point, which was 5.04 from Figure 2.
Discussion
Figure 2 indicated that the pH of the unknown acid rose steadily with the addition of sodium hydroxide up to a volume of 20.00 ml. From that point, the pH began rising sharply as it approached the equivalence point where sodium hydroxide completely neutralized the unknown acid. The pH then continued increasing steadily as the quantity of base added surpassed the equivalence point.
The equivalent weight of the unknown acid was found to be 178.76 grams while its pKa was equal to 5.05. The literature value of potassium hydrogen phthalate was 204.22 grams per mole. The disparities between the experimental figure and the known literature value could be attributed to systematic errors during the measurement of the pH values. Such errors possibly arose from inconsistencies in the usage of the pH meter or faults with the pH meter itself, which led to a pKa value that was higher than the literature value.
A pH meter with wrong calibrations could affect the pKa adversely. For example, a pH meter with a higher calibration than normal was likely to lead to higher than normal pH values hence resulting in a high pKa value. On the other hand, a pH meter with lower than the standard calibrations was likely to yield low pH and pKa values.
Errors in the measurements of the weights of NaOH and KHP could also have contributed to the differences between the literature values and experimental values. However, the trend of the titration curve was consistent with known titration curves of weak acids and strong bases. Therefore, it was concluded that titrimetric methods were useful in the determination of equivalent weights and pKa values of unknown acids.
Reference
Grossie, A D.; Underwood, K. Laboratory Guide for Chemistry; Hayden-McNeil: Plymouth, MI, 2013.