Abstract
Diffusion and osmosis are passive modes of transport that facilitate the movement of water and other molecules in living cells. Molecular kinetic energy was assessed by examining carmine in a drop of water under a microscope. The diffusion of molecules across a semi-permeable membrane was evaluated by noting the colors of solutions separated by dialysis tubing. The behavior of living cells in various environments was observed by looking at Elodea cells in hypertonic and hypotonic solutions.
The osmolarity of various solutions was also evaluated by noting the changes in weight of potato cylinders in the solutions. It was observed that water molecules were in constant, random motion and that dialysis tubing allowed the movement of water and I2KI across it. It was also noted that Elodea cells became swollen when placed in a hypotonic solution and that the osmolarity of solutions increased with an increase in solute concentration. The osmolarity of potato tubers was estimated at 0.3M. It was concluded that osmosis and diffusion were vital mechanisms in physiological processes.
Introduction
Physiological processes in the bodies of living organisms require raw materials, which when consumed lead to the production of waste substances. Consequently, it is necessary to ensure that important materials are available for metabolic processes and that the toxic waste products do not accumulate and damage cells (Hunter 72). Maintaining a steady state in living cells requires controlled movement of substances within living cells. The controlled movement of substances leads to communication between the cell and the surroundings.
The most common forms of transport in living organisms are diffusion and osmosis. Diffusion can be described as the overall movement of molecules from an area of high concentration to low concentration (Nix 165). Osmosis, conversely, is the transfer of water molecules from an area of high concentration to a low water concentration across a selectively permeable membrane (Zeuthen and Stein 205). Diffusion and osmosis are passive types of transport because they do not involve the use of additional energy in the form of adenosine triphosphate to facilitate the movement of molecules (Nix 166).
Fluids fall into three main categories based on their osmotic pressure. A hypertonic solution has a higher solute concentration than a living cell and has a tendency to draw water from cells by osmosis. A hypotonic solution has lower solute strength than a cell and tends to release water to the cell. An isotonic solution, on the other hand, is a solution with an equal solute concentration as a cell (Stoker 230). When a cell is in isotonic surroundings, water does not move in or out of the cell.
This practical aimed at investigating the attributes of molecules that enhance the progress of diffusion and the movement of solutes through a selectively permeable membrane. It was hypothesized that dialysis tubing was permeable to water molecules and impermeable to glucose and starch molecules. It was predicted that Benedict’s test would only be positive for the solution in the bag and that the same solution would test positive for the I2KI test. It was also hypothesized that the cytoplasm of a cell with a cell wall would reduce in size when placed in a hypertonic environment and increase in size when placed in a hypotonic environment. Therefore, it was predicted that if Elodea cells were placed in hypotonic environments, their cytoplasm would swell causing the cells to become turgid. It was also hypothesized that potato tubers would lose weight if placed in 0.6M sucrose.
Methods
A drop of water was placed on a glass slide. The end of a dissecting needle was used to transfer the carmine to the water droplet by touching it, putting the wet end into the carmine powder, and back to the droplet. The carmine and water mixture was stirred using the needle after which a cover slip was placed on the slide. The setup was examined under a compound microscope at low magnification followed by high magnification. The observations were recorded for later use in the discussion.
A dialysis bag was prepared by folding over 3cm at the end of a 30cm dialysis tubing pre-soaked in water. The side of the tubing was tied tightly with a thread ensuring that no liquid could leak. Equal portions of 30% glucose and starch solution were then added to the tubing through the open side. The contents of the tubing were mixed thoroughly after which the color of the solution was recorded in Table 1. 300ml of water was added to a 500ml beaker followed by a few drops of I2KI until the color of the water changed to amber-yellow.
The bag was then put into the water and I2KI solution with the unfastened side outside the beaker. The setup was left to stand for 30 minutes after which the ultimate colors of the solutions were recorded. Thereafter, the solutions were tested for the presence of sugars (Benedict’s test) by adding one dropper full of Benedict’s reagent to three test tubes filled with two pipettes of each of the solutions. The tubes were heated in a boiling water bath for approximately 3 minutes.
The two demonstration microscopes with Elodea in solutions A and B were examined under a microscope. The features that were observed were recorded in Table 2.
100ml of deionized water and various sucrose solutions were placed in labeled 250ml beakers. Seven potato cylinders that were at least 5cm long were made by making holes in potatoes using a cork awl. The cylinders were then peeled and cut to uniform lengths. Thereafter, the cylinders were wiped using paper towels and weighed to the nearest 0.01 grams. The potato cylinders were sliced into two uniform halves and placed in the beakers containing the various solutions. The setups were incubated for 1.5 to 2 hours, which included swirling of the beakers at intervals of 15 minutes. At the end of the incubation period, the weights of the potato cylinders were recorded in Table 3.
Results
The movement of carmine particles in the water was random. It was observed that the movement was continuous and did not come to a stop. Another notable observation was that tiny carmine particles appeared to shift faster than the large ones.
Table 1: Investigating the permeability of dialysis tubing to glucose, I2KI and starch
Table 2: Appearance of Elodea cells in unknown solutions A and B
Table 3: Estimating osmolarity by change in weight
Discussion
The first exercise entailed the observation of molecular movement because the molecules of gases and liquids were in continuous arbitrary movement (Mörters and Peres 7). Carmine, being insoluble in water, led to the formation of a colloidal suspension. During their motion, the water molecules collided with solid particles of carmine in what was referred to as Brownian motion. Brownian motion could bring about diffusion because it causes molecules to progress from regions of high concentration to zones of low concentration (Mörters and Peres 7). Diffusion was important in cell metabolism because it allowed cells to obtain the chemicals and molecules required for metabolic processes. For example, oxygen that was necessary for respiration reached the cell by diffusing across the cell membrane.
The ultimate colors of the solutions following the sugar test showed the movement of sugar from the bag into the beaker. The results corroborated the premise that water would move via osmosis from the beaker into the tubing. Water from the beaker moved via osmosis into the bag leading to a color change of the solution in the bag from white to deep blue, which was a positive test for starch (Harisha 44). In addition, the solution in the beaker was negative for sugars after Benedict’s test. These observations implied that there was no movement of glucose through the bag into the beaker. The findings of the experiment showed that potassium iodide had the smallest molecules followed by glucose and finally starch molecules. Supposing that the experiment began with glucose and potassium iodide in the tubing and starch in the beaker, the liquid in the beaker would change to deep blue because water would shift from the bag to the beaker via osmosis.
Based on my predictions and observations, solution A was hypertonic while solution B was hypotonic. Solution B had the greatest osmolarity because it was absorbed by the Elodea cell causing it to swell and expand. Water from a pond would be expected to be hypotonic to Elodea cells because such water contained a lower concentration of dissolved substances than those found in Elodea cells.
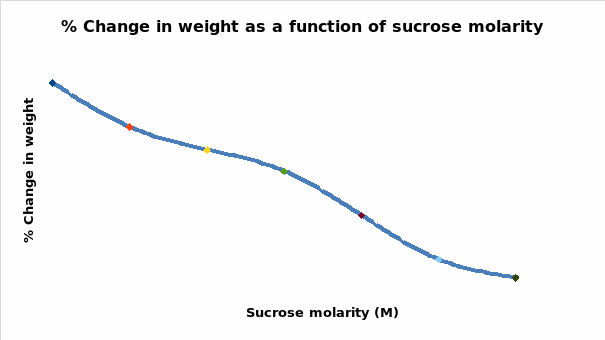
The curve intersected the zero line on the plot at a sucrose molarity of 0.3M. The data could be utilized in the establishment of the osmolarity of the potato tuber by checking the molarity where there was no net change in the weight of the tuber. Therefore, the osmolarity of the tissue was projected at 0.3M. The findings of the study confirmed the supposition that potato tubers would lose weight if placed in 0.6M sucrose.
The limitation of the experiment was that it was difficult to obtain potato cylinders with identical weights. In addition, most of the experiments involved the study of osmosis in plant cells. Future studies could look at the effects of osmosis and diffusion in animal cells.
Conclusion
Kinetic energy was necessary to facilitate the process of diffusion since no external source of energy was involved in the process. Osmosis, conversely, could only occur when an osmotic pressure existed on the two sides of a selectively permeable membrane. It was concluded that osmosis and diffusion were vital processes in maintaining the homeostasis of living cells. Therefore, to avoid any alteration in the water content of cells, it was necessary to keep them in environments whose osmolarity matched the osmolarity of the cells.
Works Cited
Harisha, S. An Introduction to Practical Biotechnology. New Delhi: Firewall Media, 2005. Print.
Hunter, G. Scott. Let’s Review: Biology, the Living Environment, New York: Barron’s Educational Series, 2009. Print.
Mörters, Peter and Yuval Peres. Brownian Motion, New York: Cambridge University Press, 2010. Print.
Nix, Staci. Williams’ Basic Nutrition & Diet Therapy14: Williams’ Basic Nutrition & Diet Therapy, St. Louis, Missouri: Elsevier Health Sciences, 2012. Print.
Stoker, H. Stephen. General, Organic, and Biological Chemistry, New York: Cengage Learning, 2012. Print.
Zeuthen, Thomas and Wilfred D. Stein. Molecular Mechanisms of Water Transport Across Biological Membranes, California: Gulf Professional Publishing, 2002. Print.