Objective
The aim of the experiment was to isolate acetylsalicylic acid, acetanilide, and sucrose fractions from Phensuprin based on their acid-base solubility differences.
Physical Properties
Acetylsalicylic Acid or Aspirin
Its synthesis involves treating salicylic acid with acetic anhydride through an esterification reaction (Harwood and Moody 132).
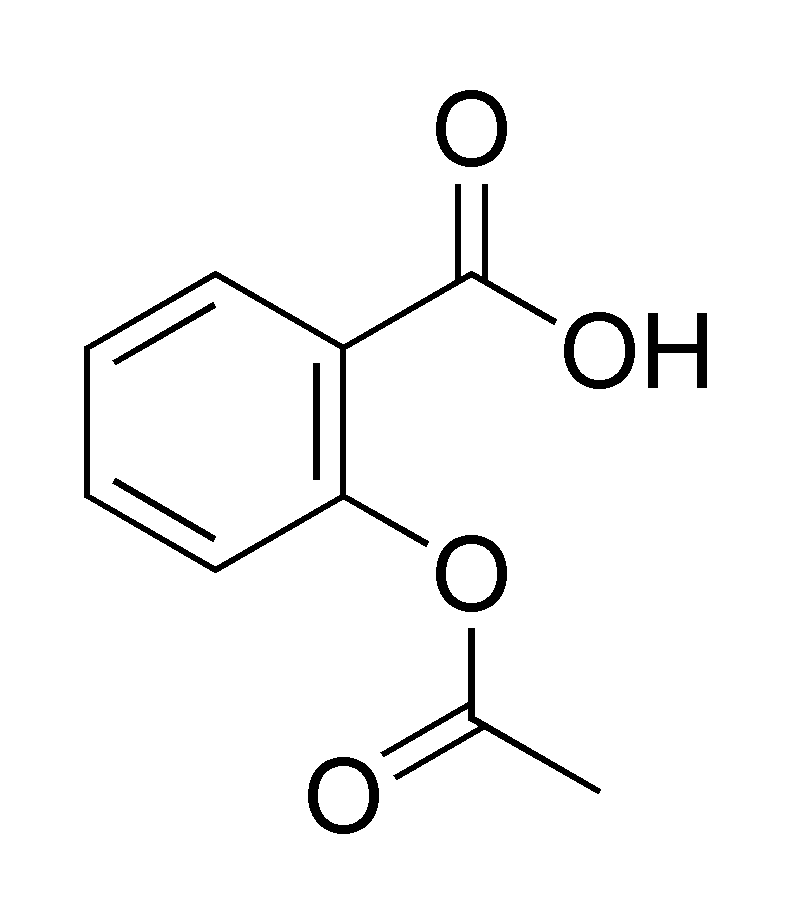
Molecular Structure
Physical state: White crystalline powder
Chemical formula: C9H8O4
Molecular mass: 180.16g/Mol
Solubility in water: 1g/100ml H2O at 37°C
Melting point: 136°C or 277 °F (with decomposition)
Boiling point: 140°C or 284 °F
Potential hazards: pain, inflammation risk, and highly flammable
Acetanilide
It is a product of acetylation of aniline (Harwood and Moody 137).
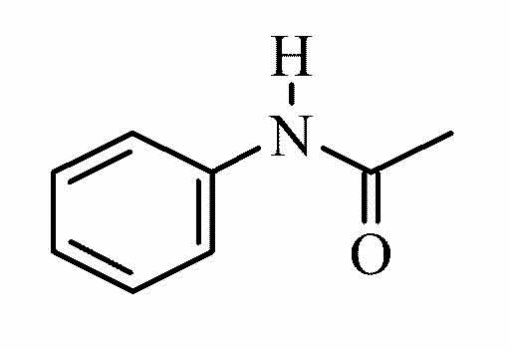
Molecular structure
Physical state: White or Grey solid with flaky texture
Chemical formula: C8H9NO
Molecular mass: 135.16g/Mol
Melting point: 114.3°C
Boiling point: 304°C
Solubility in water: 5g/L at 20°C
Density: 1.219 g/cm3
Potential hazards: potent allergen and irritant
Sucrose
It is formed through joining fructose and glucose molecules in a dehydration reaction (Harwood and Moody 145).
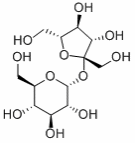
Molecular structure
Physical state: Dry white crystals
Chemical formula: C12H22O11
Molecular mass: 342.296 g/Mol
Melting point: 185.5°C
Boiling point: 320-367°F (Decomposes)
Solubility in water: 2100g/L (at 25 °C)
Potential hazards: irritant
Dichloromethane
It is a compound formed when chloride atoms substitute two hydrogen atoms in methane in the presence of UV light (Harwood and Moody 139).
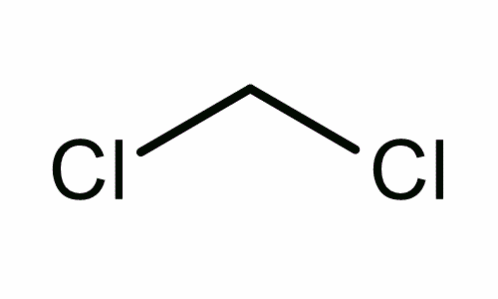
Molecular structure
Physical state: colorless liquid with “ether-like odor” (Harwood and Moody 140)
Chemical formula: CH2Cl2
Molecular mass: 84.933 g/Mol
Melting point: -95.1
Boiling point: 39.75°C at 760 mm Hg
Solubility in water: 13.2g/L at 25°C
Solubility in organic solvents: miscible with ethanol.
Potential hazards: combustible, potent carcinogen, corrosive, and poisonous.
Sodium bicarbonate
It is a compound that comprises of sodium (Na+) and bicarbonate (HCO3–) ions (Schaller par. 2).
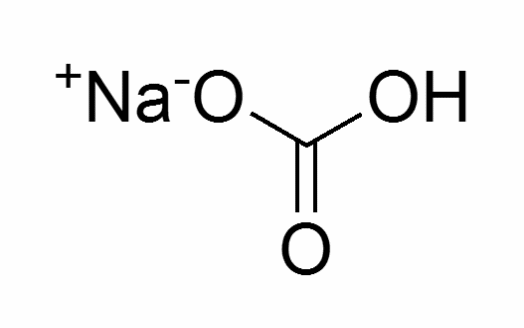
Molecular structure
Physical state: white crystalline powder
Molecular formula: NaHCO3
Molecular mass: 84.007g/Mol
Melting point: 50°C or 122°F
Boiling point: 851°C or 1,564°F
Solubility in water: 100g/L (at 25°C)
Potential hazards: irritant and poisonous
Sodium sulfate
It is a sodium compound of sulfuric acid (Schaller par. 2).
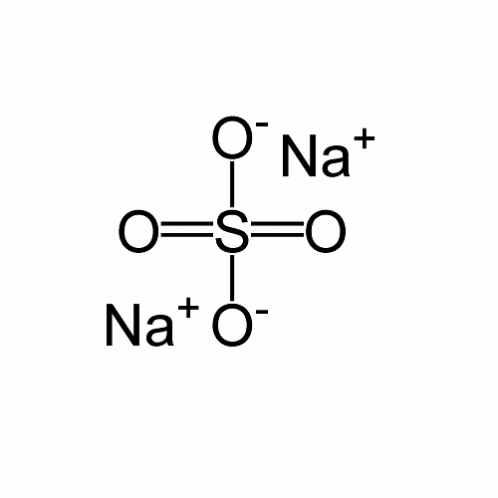
Molecular structure
Physical state: white solid or powder
Chemical formula: Na2SO4
Molecular mass: 142.042g/Mol
Melting point: 884°C or 1,623°F (anhydrous)
Boiling point: 1,427°C or 2,604°F (anhydrous)
Density: 2.664g/cm3
Potential hazards: irritant
Hydrochloric acid
It is a solution of HCl gas in water (Schaller par. 3).
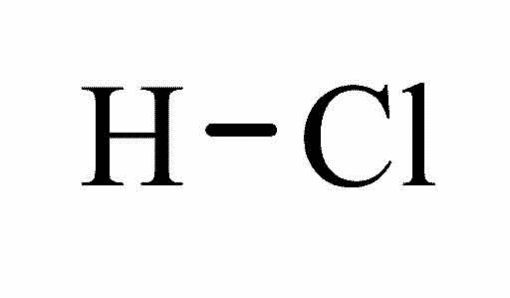
Molecular structure
Physical state: a colorless liquid with irritating fumes
Chemical formula: HCl
Molecular mass: 36.46g/Mol
Melting point: -35°C
Boiling point: 57°C
Solubility in water: 82.3g/100ml of water at 0°C
Potential hazards: highly inflammable, corrosive, irritant, and poisonous substance.
The Procedure Followed
Separation of Sucrose
To isolate sucrose in this experiment, 2g of Phensuprin, accurately measured with a weighing machine, was transferred to a clean 125ml Erlenmeyer flask and 50ml of dichloromethane added to it while stirring thoroughly to disintegrate and dissolve the solid lumps. The resulting mixture was filtered via the gravity filtration process to recover the solids on a pre-weighed filter paper. The isolated solids, i.e., Sucrose crystals, were washed gently with about 5ml of fresh dichloromethane to get rid of any unwanted salt residues. The sucrose obtained was transferred to a beaker before being air-dried for seven days at room temperature to obtain dry, dichloromethane-free crystals. Subsequently, the dried product was weighed to obtain the mass of sucrose and determine its percentage composition in 2g of Phensuprin. All the measurements and calculations were recorded for interpretation and analysis.
Separation of Acetylsalicylic Acid
To isolate acetylsalicylic acid, the dichloromethane filtrate containing acetylsalicylic and acetanilide was transferred to a clean separating funnel. The mixture was then extracted twice with 25ml aliquots of 0.5M sodium bicarbonate solution. In the first extraction, 25ml of sodium bicarbonate solution was added to the filtrate in the funnel. The plugged funnel was swirled lightly for 2 min to mix the aqueous liquids while regularly opening the stopper to depressurize the funnel. Then, the funnel was clamped on a stand to allow the aqueous portions of the mixture to separate. The bottom layer, containing the denser dichloromethane, was collected into a clean Erlenmeyer flask for the second extraction while the top layer was transferred to a labeled 400ml beaker. The dichloromethane layer was returned to the separating funnel and the second aliquot of 25ml of 0.5M sodium bicarbonate added to it. The resulting aqueous extract was combined with the first one in a beaker while the dichloromethane layer was retained for the isolation of acetanilide.
The combined aqueous extracts in a beaker were transferred to an ice/water bath to cool down. About 10ml of 6M HCl was added drop-wise with a pipette while stirring to lower the pH to less than 2. The pH of the resulting mixture was continuously monitored with a pH paper. The acidic solution was further cooled in an ice bath to allow acetylsalicylic acid to crystallize. The solid formed was recovered through vacuum filtration before washing it with 5ml of water to remove any salt residues. The acetylsalicylic acid solid was allowed to dry on a filter paper for a few minutes with the vacuum pump running. The sample was air-dried in un-stoppered vial at room temperature for seven days to dry it completely. The mass of the dry sample was used to calculate its percentage composition in Phensuprin compound.
Separation of Acetanilide
The isolation of the acetanilide component entailed the drying of the dichloromethane using anhydrous sodium sulfate. The anhydrous sodium sulfate was added in scoops to the solution while stirring to dissolve it. The solid was added until no more could dissolve in the solution. Then, the mixture was filtered and the filtrate collected in a pre-weighed 100ml round bottom flask. A rotary evaporator was then used to speed up the evaporation of methylene chloride in the filtrate leaving behind acetanilide as a white to gray solid. The solid was collected in the flask before being weighed to determine its mass and percentage composition. It was then kept in an uncorked flask for further analysis.
During the experiment, the melting and boiling points of the three Phensuprin components were measured for relative purity determination through a comparison with the respective values obtained in comparable experiments. The separation process as based on the differences in solubility and boiling/melting point between the components.
Results
The experiment separated Phensuprin into three components. The mass of each component can be used to calculate its respective percentage composition in Phensuprin.
% composition = mass of the component/Total mass of the compound
Mass of Phensuprin used = 2.0g
The mass of the filter paper used in sucrose separation = 0.98g
The mass of the filter paper + sucrose = 1.6g
Therefore, the mass of sucrose in the sample = (1.6-0.98) g = 0.62g
The mass of acetanilide obtained = 0.41g
The mass of the round bottom flask = 39.51g
The mass of acetylsalicylic obtained = 0.45g
Total mass: 0.62 + 0.41 + 0.45 = 1.48g
Total percent recovered: [1.48g/2.0] x 100 = 74%
Determination of the percentage composition of Phensuprin components
- Sucrose: [0.62/1.48] x 100 = 41.8%
- Acetanilide: [0.41/1.48] x 100 = 27.7%
- Acetylsalicylic: [0.45/1.48] x 100 = 30.4%
A Short Synopsis of the Results
Organic compounds contain more than one component. A pure sample of each component can be obtained through a separation process that utilizes the variations in the components’ chemical and physical properties, e.g., solubility. In this experiment, the components of Phensuprin were separated based on their solubility and acid-base differences. The yield from the 2g of Phensuprin used was 1.48, representing 74% of the initial sample. Thus, it can be concluded that the extraction process was a success as the amount recovered falls within the margin of error. The solid mixture recovered was separated into sucrose, acetylsalicylic acid, and acetanilide that weighed 0.62g, 0.45g, and 0.41g, respectively. The calculation of percentage composition showed that the Phensuprin sample (1.48g) contains 41.5% sucrose, 27.7% acetanilide, and 30.4% acetylsalicylic acid.
The purity of the individual components recovered was determined using their melting points. An impure compound would melt at depressed melting point (Fessenden, Fessenden, and Feist 71). The melting point of the sucrose sample was 187°C, which is near the standard value of 185.5°C. In contrast, the acetylsalicylic acid collected had a melting point of 124°C. This temperature is relatively lower than its established melting point of 136°C. Similarly, the melting point of the acetanilide sample was lower, at 100°C, than the established value of 114.3°C. Thus, the deviation from the standard melting point could be attributed to impurities in the sample.
Discussion
Extraction is a useful technique for isolating a pure compound from a mixture. The process entails separating a particular component at a time based on variations in a particular chemical or physical property, e.g., solubility. Compounds that vary in their solubility levels in an organic solvent could be separated through filtration (Padias 43). In addition, acidic or alkaline compounds are easily isolated by converting them into soluble salts and recovering them through re-crystallization. In this experiment, the components of Phensuprin, a mixture of three different compounds, was separated based on their solubility characteristics.
One of the isolated components, sucrose, is water-soluble but does not dissolve in dichloromethane, a “non-polar organic solvent” (Padias 49). In contrast, acetylsalicylic acid is soluble in this solvent but insoluble in water. Therefore, sodium bicarbonate, a basic compound, can take out the hydrogen atom from acetylsalicylic acid through a neutralization reaction to form water and sodium acetylsalicylate, a water-soluble salt that could be isolated from dichloromethane. Acetanilide is solvable in dichloromethane but not water. Additionally, this compound cannot react with sodium bicarbonate to form a water-soluble salt. The sodium acetylsalicylic salt is treated with hydrochloric acid to recover acetylsalicylic acid (C9H8O4). The two reactions are shown in the two equations below.
- C9H8O4 + NaHCO3→C9H7O4Na + HCO3-
- C9H7O4Na + HCl→C9H8O4 + NaCl
The aim of this experiment was to isolate the components of Phensuprin through acid-base extraction. The process made use of the solubility differences of the components in the polar solvent (water) and non-polar solvent (dichloromethane) to separate them. Products insoluble in either solvent remained as solids and were recovered through filtration. Phensuprin, a common painkiller, comprises three compounds, namely, acetylsalicylic acid, sucrose, and acetanilide.
In this experiment, the aim of the first extraction was to isolate sucrose from the mixture by dissolving the Phensuprin sample in dichloromethane. From the information above, it is evident that adding Phensuprin to dichloromethane would result in acetylsalicylic acid and acetanilide going into solution while sucrose, which is insoluble in organic solvents, is left behind as a solid. Therefore, to recover the sucrose, gravity filtration was used. The process entails passing the mixture through a semi-permeable filter paper that retains the solid product while allowing the liquid/filtrate to drip into a funnel (Hatti-Kaul 64). This property of the filter paper makes it useful in separating a solid-liquid mixture.
A pure sucrose product was obtained by gently washing the residue collected with dichloromethane to elute organic contaminants. This purification process helps remove impurities that may alter the chemical and physical properties of the compound (Hatti-Kaul 67). A pre-weighed filter paper was used in filtration for easy quantification of the pure residue. The filtrate contained acetylsalicylic acid and acetanilide from the Phensuprin sample. The acetylsalicylic acid was selectively isolated from the solution through the addition of sodium bicarbonate. This basic compound removes hydrogen from acetylsalicylic acid in a neutralization reaction to produce sodium acetylsalicylate salt as shown in the chemical equation below.
C9H8O4 + NaHCO3→C9H7O4Na + HCO3-
Acetanilide, being a weak base, cannot react with this compound; hence, it remains dissolved in dichloromethane solution. The sodium acetylsalicylate formed is water-soluble. Therefore, two layers are formed, an aqueous top layer and a denser dichloromethane bottom layer. Sodium acetylsalicylate, being more solvable in water than in the non-polar solvent (CH2Cl2), becomes dissolved in the aqueous layer while the hydrophobic acetanilide is retained in the dichloromethane layer. Subsequently, hydrochloric acid was added to precipitate out the compound from solution. The solid compound was then recovered through vacuum filtration.
The second extraction stage involved an acid-base reaction. The rationale was to make use of the solubility differences between acetylsalicylic acid and acetanilide to separate the two compounds. In this experiment, 25ml of 0.5M sodium bicarbonate was added in two aliquots to ensure a complete neutralization of acetylsalicylic acid to give sodium acetylsalicylate and water. The polar and non-polar solutions remained as two distinct layers. This property was utilized to separate the two immiscible solutions using a separatory funnel. The idea is to allow the liquid mixture to separate into distinct phases. The tap is then opened to release the lower layer into a beaker. The stopcock is removed during this process to equalize the external and internal pressure and avoid emulsions (Zubrick 31). This technique is based on the concept that inorganic solutes are soluble in aqueous solutions while organic compounds easily dissolve in non-polar solvents.
The delineation of the liquid mixture was due to the density differences between dichloromethane and the aqueous phase. The denser dichloromethane solution forms the bottom phase while the lighter aqueous solution floats on it. Therefore, in this experiment, the lower layer (dichloromethane + acetanilide) was released first using gravitation. It was again treated with the second portion of sodium bicarbonate to ensure that all acetylsalicylic acid in the 2g of Phensuprin is recovered. HCl was added to the aqueous extract to precipitate out acetylsalicylic acid at a lower pH and temperature. The acid donated the proton that converted the salt into an insoluble acetylsalicylic acid solid and sodium chloride (water-soluble).
To obtain acetanilide, a strong dehydrating agent (anhydrous sodium sulfate) was added to the dichloromethane solution. Anhydrous sodium sulfate has a high affinity for water molecules; hence, it is used to dry heat labile salts without heating (Zubrick 44). Any remaining dichloromethane was removed through evaporation in a rotary evaporator at room temperature. A rotary evaporator relies on partial vacuum to evaporate solvents at a lower temperature. Thus, evaporation through heating is avoided, which preserves heat-sensitive compounds.
The percentage composition of Phensuprin was calculated based on the weights of the dried compounds. The dried sucrose, acetylsalicylic acid, and acetanilide recovered from the original compound weighed 0.62g, 0.45g, and 0.41g, respectively. The percentage composition of the Phensurin sample is 41.8% sucrose, 30.4% acetylsalicylic acid, and 27.7% acetanilide. Therefore, sucrose is a major ingredient in Phensurin followed by acetylsalicylic acid. The percentage of acetanilide in the drug is the lowest among the three components.
In the experiment, a significant amount (74%) of the original sample (2g) was obtained for use in the extraction process. The purity of the isolated components was measured based on their melting points. The sucrose recovered had a melting point similar to the standard value. However, acetylsalicylic acid and acetanilide had lower melting points than standard values, indicating possible contamination with impurities. This error could have originated from the inadequate elution of dichloromethane in the first extraction, leading to contamination of acetylsalicylic acid and acetanilide. The middle layer between the aqueous and dichloromethane phase during filtration could also have contaminated the compounds.
Questions
The original organic solution was extracted two times with aqueous sodium bicarbonate. After the extraction, what compound(s) were in the organic layer? What compound(s) were in the aqueous layer?
The organic layer containing acetanilide, i.e., the bottom phase, was denser than the aqueous phase (1.33g/ml vs 1.0g/ml). The reasoning behind this conclusion is that acetanilide has a high affinity for organic solvents (dichloromethane) while inorganic salt (sodium acetylsalicylic) is easily solvable in water. Hence, sodium acetylsalicylic salt occurred in the top aqueous layer.
Assume that both acetylsalicylic acid and acetanilide are soluble in diethyl ether, and that diethyl ether was used in place of the methylene chloride. Would the ether layer be the bottom layer in the separatory funnel or the top layer? Explain your answer.
The density differences between the solvents would determine the layering of the liquid mixture. The denser of the two solvents, i.e., water and diethyl ether, and will form the bottom layer. Diethyl ether has a lower density of 0.73g/ml than that of water (1.0g/ml). Therefore, the top layer will comprise of the lighter diethyl ether floating on the bottom aqueous phase.
Assume that both acetylsalicylic acid and acetanilide are soluble in methanol, and that methanol was used in place of the methylene chloride. What problem(s) might occur during the extractions? (Careful, this is a trick question.)
Substituting methylene chloride with methanol will affect the solvability of the organic solutes. Unlike methylene chloride, methanol is not an organic solvent. Therefore, the sodium acetylsalicylate and acetanilide solution will not delineate into separate layers, as water and methanol are polar solvents. The lack of distinct layers will be a problem in separating the two components.
Historically, the solid residue that is left after the methylene chloride solution is evaporated has a lower melting point that its literature value due to impurities. Explain what impurities are present and why.
The acetanilide residue may not be completely pure because it contains traces of the compounds from earlier extraction steps. Traces of sodium carbonate, anhydrous sodium sulfate, and acetylsalicylic acid may be retained in the solid residue even after evaporation due to conjugation. The presence of impurities alters the melting point of a compound. An impure substance would melt at a temperature value lower than the standard value.
References
Fessenden, John, Joan Fessenden, and Patty Feist. Organic Laboratory Techniques. Pacific Grove: Brooks Cole Publishing, 2001. Print.
Harwood, Laurence, and Christopher J. Moody. Experimental Organic Chemistry: Principles and Practice. New York: Wiley-Blackwell, 2009. Print.
Hatti-Kaul, Rajni. Aqueous Two-Phase Systems: Methods and Protocols. New York: Humana Press, 2001. Print.
Padias, Anne. Making the Connections: A How-To Guide for Organic Chemistry Lab Techniques. Plymouth: Hayden-McNeil Publishing, 2011. Print.
Schaller, Chris. Acid-Base Extraction. 2016. Web.
Zubrick, James. The Organic Chem. Lab Survival Manual: A Student’s Guide to Techniques. New York: John Wiley & Sons, 2012. Print.