Hexokinase 2 (HK2) is an allosteric enzyme found in mammals where it is encoded by the HK2 gene. This enzyme is involved in the first stage of glucose metabolism to catalyze the phosphorylation of glucose to glucose-6-phosphate (G6P) through the addition of an ATP molecule. As such, it is considered a rate-limiting enzyme of glycolysis. HK2 is normally present in the majority of all mammalian cells, but low concentrations. This characteristic means that HK2 has low Km (10-5 M), and thus it works at maximal velocity (Vmax) at very low levels of glucose concentrations (Engelking, 2015). However, the activity of this enzyme is highly inhibited by its product (G6P), and thus it is categorized as allosteric.
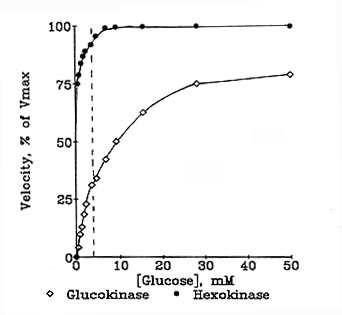
Therefore, the accumulation of G6P in cases where it is not utilized quickly leads to the reduction of HK2 activity. Additionally, HK2 is not inducible, but it is indirectly linked to insulin. Normally, the insulin allows glucose molecules to cross muscle and adipocyte cell membranes into the cells. Therefore, given that HK2 is involved in the first step of glycolysis, its expression increases in the presence of insulin.
Importance
As mentioned earlier, HK2 catalyzes the first step of glycolysis through the phosphorylation of glucose molecules by adding an ATP to form G6P. This stage of glycolysis is obligatory, and thus without it, glucose metabolism would be inhibited leading to its accumulation in cells. Through the phosphorylation process, HK2 commits glucose to the metabolism cycle thus preventing it from leaving the cells.
Additionally, the enzyme binds to mitochondria through its highly hydrophobic N-terminal, thus coupling glycolysis to the oxidative phosphorylation in mitochondria to ensure that cells get enough ATPs to meet energy demands. According to Roberts and Miyamoto (2015), HK2 maintains cell integrity and survival by “exerting antioxidant effects, direct protection of mitochondria against stress, and facilitation of autophagy under starvation” (p. 249). Therefore, HK2 plays an important role in glucose metabolism to ensure that cells’ energy demands are met, and it helps in ensuring cell survival.
Expression of HK2 by Normal and Cancer Cells
In normal cells, HK2 is expressed in low concentrations, especially in liver and muscle cells where it plays an important role in glycolysis. This enzyme is regulated through a feedback mechanism whereby it is inhibited by its product (G6P). However, the available research shows that HK2 is up-regulated in cancer cells (Anderson, Marayati, Moffitt, & Yeh, 2016; Lee et al., 2016; Hoppe-Seyler et al., 2017). One of the major characteristics of tumor cells is the ability to outcompete normal cells in the metabolism of glucose through glycolysis. This attribute explains why cancer cells can metastasize and outgrow normal cells.
According to Hoppe-Seyler et al. (2017), cancer cells have been shown to have increased expression of HK2, which leads to the Warburg effect whereby even under aerobic conditions, such cells prefer glycolysis to the commonly used pathway of oxidative phosphorylation used by normal cells. The Warburg effect is closely linked with the increased expression of HK2. Cancer cells undergo reprogramming to “increase the rate of aerobic glycolysis (‘Warburg effect’) for the generation of energy (ATP) and intermediates for tumor cell growth (e.g. nucleic acid precursors, lipids)” (Hoppe-Seyler et al., 2017, p. 106342). HK2 plays a key role in regulating and rate-inhibition of this process.
The connection between HK2 and Hypoxia
Under aerobic conditions, normal cells use oxidative phosphorylation to produce ATPs to meet cellular energy demands. However, cancer cells prefer glycolysis for the generation of energy molecules whether under aerobic or anaerobic conditions, under the activity of HK2. According to Menendez, Teygong, Wade, Florimond, and Blader (2015), the gene that regulates the expression of HK2 enzyme is regulated by hypoxia-inducible transcription factor 1 (HIF-1). This assertion implies that when cells experience oxygen tension HIF-1 triggers the HK2 gene to express the HK2 enzyme, which in turn catalyzes the phosphorylation of glucose leading to glycolysis.
Hypoxia plays an important role in the growth and spread of cancer cells. A study by Bhalla et al. (2018) showed that under hypoxic stress, cells selectively favor the translation of the HK2 gene as its “expression was highly induced in lymphoma cell lines compared with a normal B cell line GM02184 and primary B-cells derived from normal lymphoid tissue” (p. 8). Therefore, these studies underline an important mechanism through which cells experiencing hypoxia respond through the up-regulation of the HK2 gene. Ultimately, hypoxia leads to increased levels of enzyme HK2, and thus glycolysis is facilitated through the phosphorylation of glucose molecules into G6P.
The connection between HK2 and Mitochondria
HK2 binds to mitochondria through the voltage-dependent anion channel (VDAC), which is the pore-forming membrane on the outer surface of mitochondria. Such bound HK2 uses ATPs generated in the mitochondria as a substrate for initiating glycolysis by phosphorylating glucose molecules into G6P. According to Roberts and Miyamoto (2015), the binding of HK2 to mitochondria initiates a cascade of activities that lead to the inhibition of apoptosis, thus conferring protective effects to cells.
HK2 binding to VDAC on the surface of mitochondria leads to the closure of mitochondrial permeability transition pore (mPTP), which is a pathological pathway that allows the influx of solutes into the mitochondria. This channel is proapoptotic, and thus its closure leads to cellular protection. The capacity to protect cells against apoptosis implies that HK2 improves the survival chances of the affected cells and as mentioned earlier, cancer cells have elevated levels of HK2. Therefore, HK2 is an important enzyme in the growth and metastasize of cancer cells because it protects them from self-destruction, thus bypassing the mechanism of most immune cells.
Inhibition of HK2 and its Role as Potential Cancer Treatment
HK2 could be inhibited through different ways to prevent its glucose phosphorylation characteristics and the mitochondria binding mechanism. For example, the oncogene Kras facilitates the expression of the HK2 gene, and thus its down-regulation would have the same effects on HK2 (Wang et al., 2016). In cancer cells, the Warburg effect plays a central role in ensuring that glycolysis takes place through the phosphorylation characteristics of HK2. Additionally, HK2 increases the survival rates of tumor cells by inhibiting apoptosis. According to Seo, Crochet, and Lee (2014), 3-Bromopyruvate is a strong inhibitor of HK2, and it functions through the covalent modification of sulfhydryl groups in the enzyme, which causes its dissociation with VDAC on the surface of mitochondria.
Therefore, inhibition of HK2 can be used as a potential cancer treatment in two ways. First, inhibiting the activity of HK2 means that glycolysis will not occur through the phosphorylation of glucose molecules to form G6P. As such, cancer cells will not get enough ATPs to meet their energy requirements, and thus they will not metastasize. Second, dissociating HK2 from mitochondria reverses the anti-apoptosis properties of cancer cells, and thus they can die through self-destruction. Ultimately, cancer cells will die and be eliminated from the body, which is the objective of cancer treatment.
Application
Given the benefits associated with the inhibition of HK2 in the management of tumor cells, this technique could be applied in chemistry, biology, and medicine as part of cancer therapy. In chemistry, this knowledge could be applied to come up with substances that inhibit the activity or expression of HK2. These substances could be developed further into drugs, which could be useful in the field of medicine in the treatment of different forms of cancer.
In biology, studying and exploiting the activity of HK2 would play an important role in the understanding of how different mechanisms could be used to inhibit the activity of the enzyme and the expression of its gene in cancer therapy. In summary, understanding how HK2 functions are important in chemistry, biology, and medicine as the three areas of study converge to create cancer treatment regimens.
Conclusion
HK2 enzyme catalyzes the phosphorylation of glucose molecules into G6P as the first step of glycolysis. This enzyme exists in almost all body cells, but its expression is mainly found in musculoskeletal and hepatic cells. HK2 is important because glycolysis plays a central role in the provision of enough energy molecules. Under hypoxia, the HK2 gene is up-regulated as a survival mechanism during oxygen stress. HK2 also binds to mitochondria to prevent apoptosis, thus increasing the survival rate of cancer cells. Therefore, inhibiting the activity of the HK2 enzyme and expression of its gene starves cancer cells and allows apoptosis. Consequently, this technique can be used in the treatment of different types of cancer.
References
Anderson, M., Marayati, R., Moffitt, R., & Yeh, J. J. (2016). Hexokinase 2 promotes tumor growth and metastasis by regulating lactate production in pancreatic cancer. Oncotarget, 8(34), 56081-56094.
Bhalla, K., Jaber, S., Nahid, M. N., Underwood, K., Beheshti, A., Landon, A., … Gartenhaus, R. B. (2018). Role of hypoxia in diffuse large B-cell lymphoma: Metabolic repression and selective translation of HK2 facilitates the development of DLBCL. Scientific Reports, 8(1), 1-15. Web.
Engelking, L. R. (2015). Textbook of veterinary physiological chemistry (3rd ed.). San Diego, CA: Academic Press.
Hoppe-Seyler, K., Honegger, A., Bossler, F., Sponagel, J., Bulkescher, J., Lohrey, C., & Hoppe-Seyler, F. (2017). Viral E6/E7 oncogene and cellular hexokinase 2 expression in HPV-positive cancer cell lines. Oncotarget, 8(63), 106342-106351.
Lee, H. G., Kim, H., Son, T., Jeong, Y., Kim, S. U., Dong, S. M., … Park, J. H. (2016). Regulation of HK2 expression through alterations in CpG methylation of the HK2 promoter during the progression of hepatocellular carcinoma. Oncotarget, 7(27), 41798-41810.
Menendez, M. T., Teygong, C., Wade, K., Florimond, C., & Blader, I. J. (2015). siRNA screening identifies the host hexokinase 2 (HK2) gene as an important hypoxia-inducible transcription factor 1 (HIF-1) target gene in Toxoplasma gondii-infected cells. mBio, 6(3), 1-11. Web.
Roberts, D. J., & Miyamoto, S. (2015). Hexokinase II integrates energy metabolism and cellular protection: Akting on mitochondria and TORCing to autophagy. Cell Death and Differentiation, 22(2), 248-257.
Seo, M., Crochet, R. B., & Lee, Y. -H. (2014). Targeting altered metabolism: Emerging cancer therapeutic strategies. In S. Neidle (ed.), Cancer drug design and discovery (pp. 427-448). San Diego, CA: Academic Press.
Wang, H., Wang, L., Zhang, Y., Wang, J., Deng, Y., & Lin, D. (2016). Inhibition of glycolytic enzyme hexokinase II (HK2) suppresses lung tumor growth. Cancer Cell International, 16(9), 1-11. Web.