Abstract
The objective of this experiment was partly to determine the behaviour of PH curve of a triprotic acid and hence determine its pKa value (s), and on the other part to determine the concentration of an analyte. As such, 10 ml. of 0.1 M. H3Po4 acid was reacted with a titrant- 0.1 M. NaOH. The PH of the mixture was taken at equal intervals of volumes (0.5 ml.) sequentially. This was recorded concurrently with the volume after which a PH-volume graph was plotted for analysis. On the other hand, for the strong acid titration, 0.01 M. NaOH was titrated against a 20 ml hydrochloric acid.
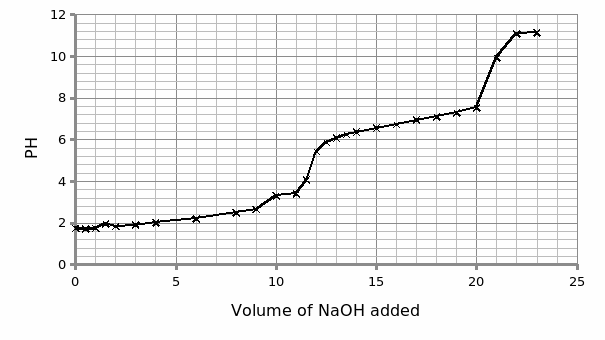
The trend of the graph revealed that phosphoric is a triprotic acid giving pKa values (2.3 and 7.0) which gave consistent results with the theoretical values. The third pKa value was not established because the data collected was limited.
Introduction
Biological reactions are enzyme catalysed hence require optimum PH to function efficiently. Tissues deprived of oxygen always create a toxic environment that ruins the cells due to metabolic acidosis. To curb this, buffer solutions come in handy to maintain the PH of the cells within an optimal range (Perrin, Dempsey & Serjeant, 1981).
Weak acids always dissociate to their respective conjugates: conjugate acid and conjugate base. This can be shown by the equation: HA↔H++A–. The ability of an acid to dissociate depends on the equilibrium dissociation constant, Ka. Thus, the higher the value the greater is the affinity to dissociate hence the stronger the acid.
Noteworthy, by definition, the PH of a solution is the negative log of H+ concentration. On the other hand, pKa=-logKa. This is to say that the pKa is inversely proportional to the strength on an acid. A combination of these equations takes us to Handerson-Hasselbalch Equation: PH=pKa+log [A–]/ [HA].
Objective
The objective of this experiment was partly to determine the behaviour of PH curve of a triprotic acid and hence determine its pKa value (s), and on the other part to determine the concentration of an analyte.
Procedure
For this experiment, 10 ml. of 0.1 M. H3Po4 acid was reacted with a titrant- 0.1 M. NaOH. The PH of the mixture was taken at equal intervals of volumes (0.5 ml.) sequentially. This was recorded concurrently with the volume after which a PH-volume graph was plotted for analysis. Before this, strong acid titration was also performed in part 1 of the experiment where 0.01M. NaOH was titrated against a 20 ml hydrochloric acid. The objective being to determine the concentration of HCL used determined by the end point courtesy of a colour change by phenolphthalein indicator.
Results
Concerning the experimental data, the below curve was obtained.
Discussion
With regards to strong acid titration, the below calculation will reveal the concentration of the analyte:
Concentration of the analyte (Ca) =moles of the analyte/volume of the analyte.
But moles of the analyte=moles of the titrant.
Moles of the titrant= volume of the titrant, Vt multiplied by concentration of the titrant, Ct. = 0.01Vt.
Therefore, moles=volume of the analyte* concentration of the analyte= 20/1000*(0.01Vt/1000). = 0.002Vt.
Therefore, Ca= 0.002Vt/0.02 =0.1Vt Molar.
The experimental graph depicted a standard curve synonymous to a polyprotic acid. In particular, the curve is a triprotic curve since it has three pKa values. PH is a gauge of the degree of acidity or basicity in a given aqueous solution. On the other hand, pKa defines the strength or weakness of an acid or a base.
When doing strong acid/ strong base titrations, the objective is to determine the concentration of unknowns: either base or acid. The identity of one solution needs to be known to identify the other’s concentration.
A buffer solution is a solution that is less responsive to addition of either small volumes of an acid or an alkali (Scorpio, 2000). This is vital in the stability of PH levels.
Using PH curves for buffer solutions, one can tell the range over which the PH works best. This as portrayed by the sketches above is the region(s) with flat or gradual gradient. The pKa values for phosphoric acid are 2.3 and 7.0.
The acid used for this experiment is an example of a polyprotic acid (triprotic) hence, it dissociates in three steps represented by the equations below:
The first ion of hydrogen breaks off i.e., H3Po4 →H++H2Po4–,
The second ion dissociates from H2Po4– i.e., H2Po4–→H++HPo42-,
The third ion dissociates from HPo4– i.e., HPo42-→H++Po43-
As such, the graph exhibit alternates three near-plateau and spike ups trends associated with the stepwise dissociations. Thus, the curve has three pKa: Pk1, Pk2 and Pk3. The acid’s behaviour is akin to that of weak acids (Kenkel, 1994).
An indicator as used in titrations acts as a weak acid hence once in a reaction, and upon addition of enough base or acid, it dissociates eventually exhibiting a corresponding colour change reserved for either base or acid. If PpH is a phenolphthalein indicator, it changes to pink in an acid upon dissociation as shown in the equation: PpH→Pp+H+. The same approach can be used in the alkali reactions. (Atkins & Jones, 2008).
The carbonate buffer system in human body strives at maintaining a relatively steady PH in the blood within small variations in hydrogen ions concentration lest the cells undergo dysfunction. This is achieved courtesy of the following equations:
- CO2(g) <–> CO2(aq)
- CO2(aq) + H2O(l) <–> H2CO2(aq)
- H2CO3 <–> H+ + HCO3–
- HCO3- <–> H+ + CO32-
As such, an increase in H+ ions in blood initiates a reverse reaction starting from the last equation to the first one. In the contrary, a deficiency in the same initiates a forward reaction thus maintaining the body PH within the recommended range (Hulanicki, 1987).
Conclusion
The objective of this experiment was met since it was established that phosphoric is a triprotic acid giving pKa values (2.3 and 7.0) which gave consistent results with the theoretical values.
References
Albert, A., & Serjeant, E.P. (1971). The Determination of Ionization Constants: A Laboratory Manual. Kansas City: Chapman & Hall.
Atkins, P.W., & Jones, L. (2008). Chemical Principles: The Quest for Insight (4th Ed.). Vatican City State: W.H. Freeman.
Department of Chemistry. (2008). Redox Reactions. Web.
Hawthorne, A., & Thorngate, J. (1979). Application of Spectroscopy. Birmingham City, UK: University of Alabama.
Hulanicki, A. (1987). Reactions of acids and bases in analytical chemistry. New York City: McGraw-Hill Companies Inc.
Kenkel, J. (1994). Analytical Chemistry for Technicians. Boca Raton, US: Lewis Publishers.
Perrin, D.D., Dempsey, B., & Serjeant, E.P. (1981). pKa Prediction for Organic Acids and Bases. Kansas City: Chapman & Hall.
Reichardt, C. (2003). Solvents and Solvent Effects in Organic Chemistry: Solvent Effects on the Position of Homogeneous Chemical Equilibria. New York City: John Wiley & Sons, Inc.
Scorpio, R. (2000). Fundamentals of Acids, Bases, Buffers & Their Application to Biochemical Systems. Birmingham City, UK: University of Alabama.
Sigurds, S. (1986). Applications Of UV-Visible Number UV-31 Derivative Spectrophotometry. Steinhauserstrasse, Switzerland: Peter Lang Publishing Group.
Skoog, D., West, D., & Holler, F. (1992). Fudamentals of Analytical Chemistry. Fort Worth, US: Saunders College Publishing.
Skoog, D.A.; West, D.M.; Holler, J.F.; Crouch, S.R. (2004). Fundamentals of Analytical Chemistry (8th ed.). Salt Lake City: Thomson Brooks/Cole Publishers.
Steiner, J., Termonia, Y., & Deltour, J. (1972); Analitical Chemistry. Geneva: ILO Publications.
Szalay, L. (2008). Atomic Absorption Spectrophotometry (AAS). Budapest, Hungary: Petrik Lajos Publications.
Vandenbelt, J., & Henrich, C. (1953). Application Spectroscopy. Geneva: ILO Publications.