Introduction
The objective of the experiment is to determine the influence of salt concentration, potassium chloride (KCl), on the solubility of potassium bitartrate (KHT). Moreover, the experiment aims to compare the effect of magnesium sulfate and glucose on the solubility of KHT. Normally, the solubility of a salt is dependent on the presence of other salts or molecules in a solvent. The solubility product constant offers an equilibrium point of an electrolyte dissolved in a certain solvent. For instance, when a salt named MN dissolves in a solvent, it forms a solution with its ions and solid particles at an equilibrium point called product solubility constant. For example, the product solubility constant (Ksp) for salt MN(s) is [M+] [N–].
MN(s) ⇋ M+ (aq) + N– (aq)
Solids and liquids do not form part of equilibrium expressions because they do not participate in chemical reactions of ionic formation. The concentration of solids does not influence the concentrations of cations and anions in a given solution. So long as an adequate amount of solid is present in a solvent, the concentration of ions would be dependent on the solubility rather than the concentration of solid. Since the concentration of liquids is very high when compared to solids, it does not influence the concentration of ions. In this case, the experiment focuses on the solubility of KHT, which is a salt that forms K+ and KH– ions when it dissolves in appropriate solvents.
KHT(s) ⇋ K+(aq) + HT– (aq)
When KHT dissolves in water or solutions, it generates HT–, which is an anion that forms a weak acid. In this view, titration of HT– with potassium hydroxide provides the number of moles of KTH, and thus, its solubility in a given solvent.
A large equilibrium constant means that a solute is very soluble in a solvent, whereas a small equilibrium constant implies that a solute has low solubility in a solvent (Boikess 53). Essentially, large and small equilibrium constants show that products and reactants dominate a solution respectively. The product of cations and anions of a dissolved salt represents the solubility product constant of a certain solute. Comparatively, molar solubility is the maximum amount of solute in moles that can dissolve in a liter of solvent at the point of saturation. According to Le Chatelier’s principle, a disturbance of equilibrium causes a reaction to shifting right or left to stabilize the system (Kotz, Treichel, Townsend, and Treichel 481). Thus, the addition of reactants or reduction of products would cause the equilibrium to shift to the right, whereas the addition of products or reduction of reactants would cause the equilibrium to shift to the left.
The titration method enables the determination of the solubility of KHT in various solvents. Based on a known volume and concentration of solutions, the titration method allows the calculation of moles of an unknown solution. Since HT– a weak acid, phenolphthalein is a pH indicator for it appears colorless in acid and pink in basic solution. The endpoint of the titration depicts the neutralization point where the acid and base react to form salt and water as the only products. Given that molar solubility is the amount of moles that can dissolve in a liter of solvent, titration with a known amount of solvent and stoichiometry permits its determination. The solubility product constant is calculated by measuring the concentration of anions and cations in a solution and determining their product.
Procedure
Pure water and solutions of KCl were used to determine the solubility of KHT. Pure water was obtained through the process of distillation while 0.1M KCl was prepared by weighing 7.45g and dissolving in a liter of distilled water. Moreover, 0.05NaOH was prepared by weighing 2g and dissolving in a liter of water. Three types of solvents, namely, 100ml of pure water, 100ml of 0.05M KCl (50ml of water + 50ml of 0.05M KCl), and 100ml of 0.1M KCl were added into three separate conical flasks. Subsequently, 1g of KHT was measured and added into each of the conical flasks to determine their solubility in the three solvents. A magnetic stirrer was used to mix the contents of the flasks for 20 minutes and undissolved KHT was removed using a filter paper. A volumetric pipette was used to measure and transfer 25ml of the filtrate into the flask where titration was done with 0.05M NaOH using three drops of phenolphthalein as the indicator of the endpoint. To enhance the accuracy of the findings, titration was performed twice for each solution and the findings averaged.
Results
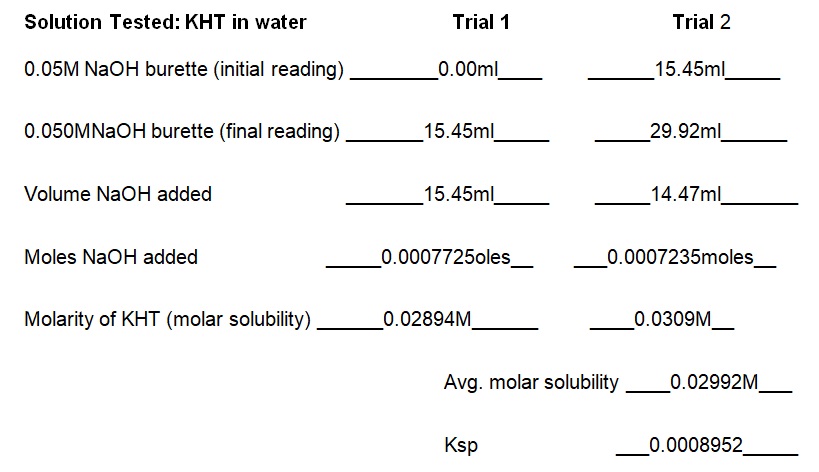
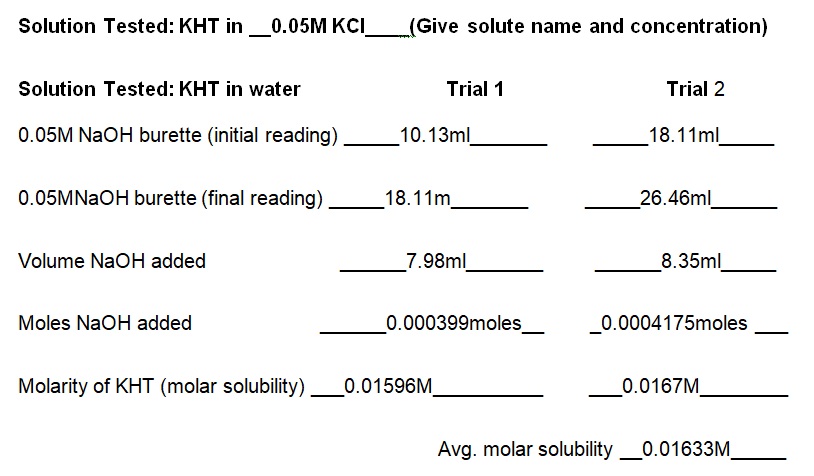
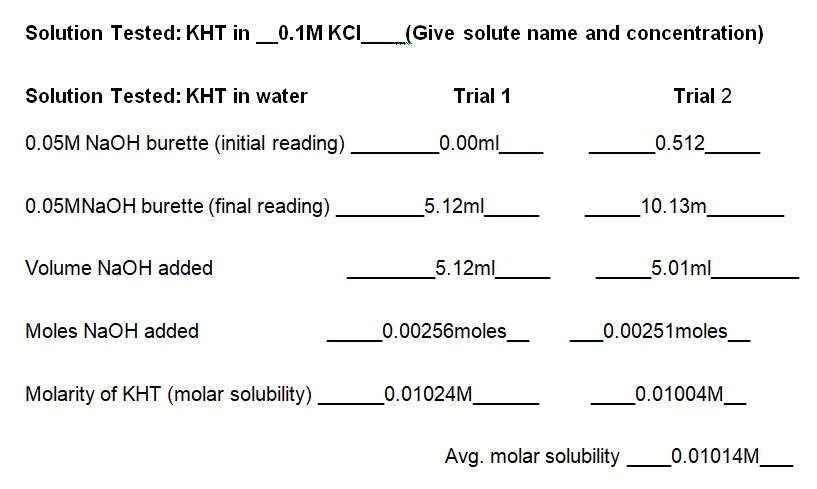
Report Sheet for Lab Results
Calculations
Table 1.Calculations of Moles and Molar Solubility.
Table 2. Summary of the Effect of Solutes on Solubility of Salts.
Table 3. Summary of Class’ Results.
Discussion
The experiment demonstrated that the concentration of KCl in the solution determines the solubility of KHT. Essentially, the solubility of KHT decreases as the concentration of KCl increases. Results of the experiment (see table 1) indicate that the solubility values of KHT in water, 0.05M KCl, and 0.1M KCl are 8.952 × 10-4 moles/L, 2.67 × 10-4 moles/L, and 1.0282 × 10-4 moles/L respectively. KCl decreases the solubility of KHT because it competes for water molecules and reduces the amount available for the solvation of additional ions (Yizhak 45). As per Le Chatelier’s principle, KCl increases the number of reactants and causes the reaction to shift right. Since KCl was added earlier than KHT, it saturated the solution and prevented the dissolution of more ions in water. Thus, the accumulated products of KCl prevented KHT from forming further products at a given equilibrium point. Fundamentally, KHT is a salt with a molecular mass of 188.76g and solubility of 5.7g per liter at room temperature (Ebbing and Gammon 65). In terms of grams per liter, the solubility values of KHT in water, 0.05M KCl, and 0.1M KCl are 5.625g/L, 3.082g/L, and 1.914g/L correspondingly (see table 2). Thus, a comparison of the solubility constant in the literature (5.7g/L) to the experimental one (5.625g/L) does not show a substantial difference.
Observations made on the class’s results show that the solubility of KHT varies according to the type and concentration of solutes (see table 3). KHT exhibited the highest solubility in MgSO4 followed by water, glucose, and KCl, as indicated by the average volumes of NaOH required to reach the endpoint of the titration. When the concentration of KCl doubles, it causes the molar solubility of KHT to decrease by about half. In contrast, MgSO4 appears to increase the molar solubility of KHT. However, glucose has no significant effect on the molar solubility of KHT for the volume required to reach the endpoint does not deviate considerably from that of water. Since water has no ions that compete for solvation, the solubility of KHT is average. The similarity of ions in KCl and KHT decreases the solubility because their ions compete for water molecules. The solubility of KHT in MgSO4 was higher than that of water due to the divalent ions and reactivity. As ions of MgSO4 are divalent, they react with the double amount of moles of KHT. Additionally, as magnesium is less reactive than potassium, a displacement reaction, which favors the right shift occurs and increases the solubility of KHT. Glucose has no effect on the solubility of KHT for its solution lacks ions, which compete for water molecules during solvation.
Two major errors in the experiment are parallax error and titration error. As the experiment entailed the measurement of 100ml of liquids, 25ml of solution for titration, and titration volume, parallax error is prone to occur. Specifically, reading of measurements from an elevated level would lead to under measurement. Therefore, under measurement of volumes would decrease the calculated molar solubility of KHT. Additional titration errors could occur due to the inability to determine the correct endpoint of the titration. Gilbert and Martin explain that the faint color of the endpoint is not persistent and definite (484). In this view, one is tempted to add excess NaOH to observe clear color change, which occurs at pH 8 rather than at the neutral pH 7. Thus, the addition of excess NaOH would increase the calculated molar solubility of KHT.
Works Cited
Boikess, Robert. Chemical Principles for Organic Chemistry. Cengage Learning, 2014.
Ebbing, Darrell, and Steven D. Gammon. General Chemistry. Cengage Learning, 2016.
Gilbert, John, and Stephen Martin. Experimental Organic Chemistry: A Miniscale & Microscale Approach, 6th ed., Cengage Learning, 2015.
Kotz, John, Paul Treichel, John Townsend, and David Treichel. Chemistry & Chemical Reactivity, 9th ed., Cengage Learning, 2014.
Yizhak, Marcus. Ions in Solution and Their Solvation. John Wiley & Sons, 2015.