Introduction
Acetone is one of the most widely used solvents in the world and can be used as an intermediate for several commercial products such as acrylic plastic, bisphenol, polycarbonates and epoxy resin, paints, and adhesives among others. According to Tremoulet et al. (Para 2), Acetone is often a by-product of phenol, with scientists capable of producing acetone from a variety of materials including propylene, cumene, and isopropanol.
In industrial production acetone is an organic compound with a chemical formula of (CH3)2CO. Tremoulet et al. affirm that during production of cumene, “hydro peroxide generated from this process undergoes slicing to engender phenol and acetone” (Para 2).
Where scientists require a high concentration of high-purity acetone, they can undertake Catalytic dehydrogenation of isopropanol as a substitute method to manufacturing acetone. As a fresh engineer and based on knowledge in chemical engineering, this paper seeks to design an acetone plant that produces 50 thousand tons per year from isopropyl alcohol.
Problem Statement
For several decades, scientists have consistently tried to incorporate knowledge to individuals on how to produce simple acetone, with the importance of acetone becoming widely known. A research documented by Rahman indicates, “The catalytic hydrogenation of acetone is an important area of catalytic process to produce fine chemicals” (113).
The fine chemicals are useful since the form key element in the conversion of liquid or gaseous fuels as well as having important application in the heat pumps. Typically, scientists have developed vast number of homogeneous complexes and heterogeneous catalysts that have existed until the date for the production fine chemicals, with little knowledge existing in the production of acetone using isopropyl alcohol (IARC 479).
For this reason therefore, this paper seeks to provide a comprehensive report on the chemical production of acetone using isopropyl alcohol as the main material.
Physical properties of all components
Acetone is an organic compound having several physical properties during the entire process of production. Following the procedure in the production of acetone, the several materials emerge during and after the production. To begin with, in the feed drum there are normally three materials in this mixture.
The mixtures found in the feed drum as described in the production process entail recycle stream, water, and isopropyl alcohol. This mixture has a number of physical properties noted as colourless, moveable, with mildly pungent smell and liquid in nature (IARC 481).
The mixture goes through all other procedures including processes in the vaporizer, heater and reactor the mixture maintains similar physical characteristics. However, flammability of the mixture is eminent throughout the process and engineers should take caution during the chemical production of acetone.
In the final process, the mixture produces acetone, as the final product and this liquid possesses characteristics such as colorlessness, mobility, flammability, and pungency in its smell.
Production process and alternative processes
In the process of producing acetone-using isopropyl alcohol, several processes are inclusive in this procedure. Since the advent of discovery of production of acetone, several Chemical Engineering processes and procedures have emerged with a range of materials proving imperative. As mentioned before, acetone can result from the chemical reaction of several materials.
Laboratory production of Acetone can use isopropyl alcohol as the reactant; can use oxidation of Propylene process, oxidation of Butanol, oxidation of Isopropyl Benzene as well as Dehydrogenation of Isopropanol (Arda et al. 8). During the production or engineering process of acetone, all chemical reactions involved are essential to produce fine acetone, which is useful commonly as a solvent.
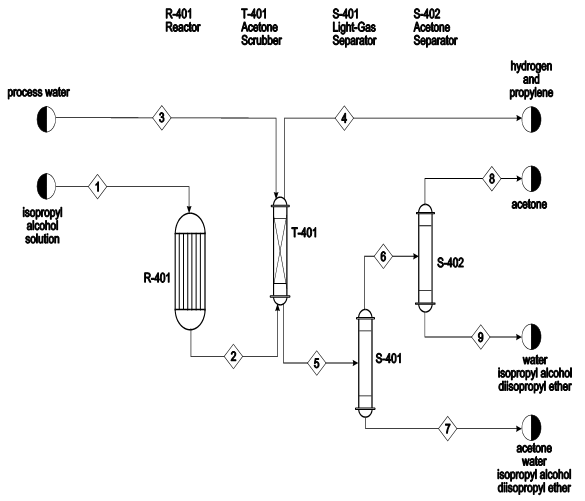
To understand the chemical production of acetone, this study provides the entire engineering process of acetone from isopropyl alcohol using “feed drum, vaporizer, heater, reactor, furnace, cooler, condenser, flash unit, scrubber, acetone and IPA columns” (Arda et al. 10). The study will also provide a diagrammatic presentation of the process.
Production process
In common processes, original invention of acetone instigates in the feed drum where mixing of feed including isopropyl alcohol, water, and recycle stream takes place. Vigorous mixing of these feeds (components) leads to a substantial reaction. From the feed drum, the mixture goes to another important stage, where the vaporizer provides room for further chemical reaction (Arda et al. 11).
The mixture in the vaporizer changes the streams phase into vapor. After undergoing the necessary procedures required in the vaporizer, the mixture undergoes another important phase in the heater. In the heater chamber, the mixture undergoes vigorous heating to enhance reaction through subjecting the mixture to higher temperatures.
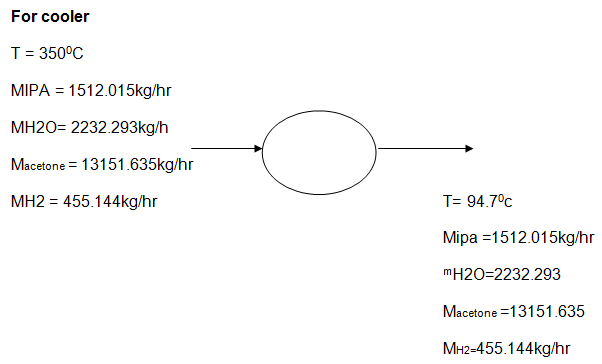
Reactor is another important section, where this section provides tubular flow reaction. In the reactor, the process is capable of achieving two products namely, Acetone, hydrogen gas, while isopropyl and water undergo discharging. A concentrated mixture comprising of hydrogen, water, acetone, and isopropyl-alcohol proceeds to the cooler and then further to the condenser unit before it proceeds to the flash unit.
In the flash unit Hydrogen, acetone, isopropyl-alcohol and water form the top product. The top product comprising of acetone proceeds to another important procedure in the scrubber. In the scrubber chamber, the main purpose is to separate hydrogen gas from the mixture.
Thereafter, the bottom product produced in the flash unit comprising of water, isopropyl alcohol, and acetone is mixed with the bottom product from the scrubber in the acetone column. In the acetone column, the reaction produces acetone from the top product with almost 99-wtpercentage isopropyl and water (Arda et al. 7). 0.1% of acetone then proceeds to isopropyl alcohol column from the bottom product.
Finally, the top product of the isopropyl column is taken to the feed drum. The remaining part of mixture (residue) found, popularly referred as bottom product, remains as dissipate of the reaction. The following is the general formulae for the reactions in the production of acetone using isopropyl alcohol.
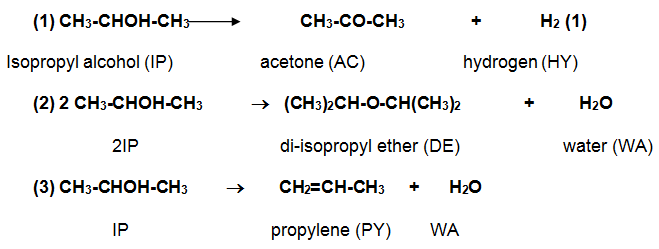
Discussion of the production process
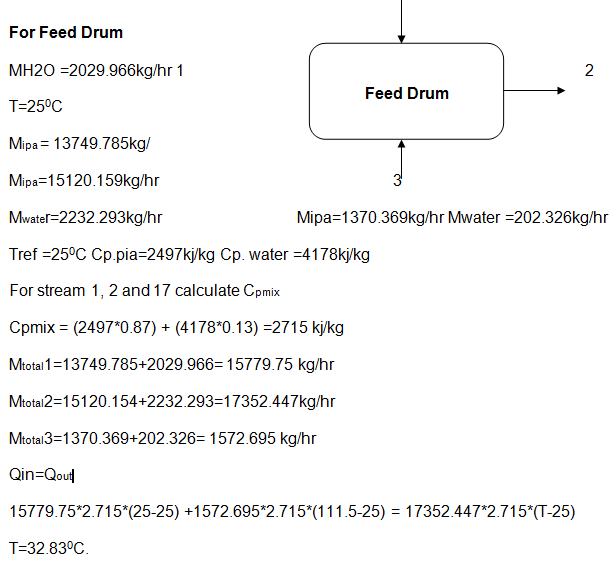
In the feed drum: A feed is a tank like object used in the mixing of the recycle stream and the feed stream. The mixture ran at a temperature of 25 Degrees Celsius in the feed stream, which the engineers assumed it was constant. The temperature in the recycle stream used was about 110 degrees Celsius.
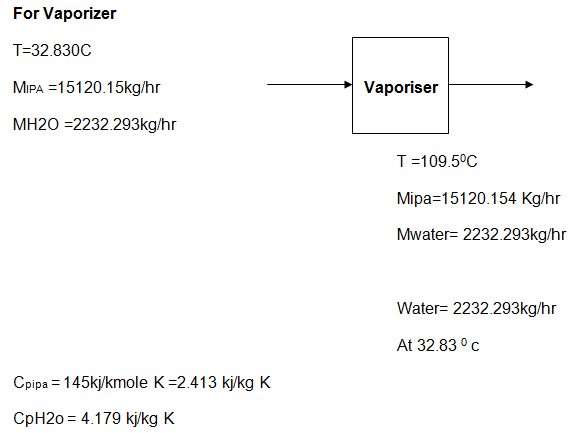
In the vaporizer: to reduce the chemical reaction speed necessary in the fine production acetone, it was important to consider the use of salt. Engineers used similar temperature of the mixture leaving the feed drum to maintain the reaction in the vaporizer. The mixture undergoes several chemical reactions to produce the product known as acetone.
Kinetic data of the reactions
During the chemical production process of acetone energy consumption and the rate of consumption are important factors considered in a bid to obtain fine acetone. This report adopted the kinetic energy data provided by (Arda et al. 15) to explain the energy consumption of the process.
This part covered energy data consumed in the Feed Drum, where the process begins to the isopropyl-alcohol column, where the process ends.
Other related processes (Chemical processes)
Oxidation of Propylene-as postulated before, several other processes can be useful in production of acetone. Production of acetone can involve oxidation of Propylene. In normal occasions, a process for production of acetone by direct oxidation of propylene, involves the oxidation process by air. The process principally involves a solution of copper chloride with small quantities of palladium chloride mixed in the air to produce acetone.
Chemical formulae: C3H6 + 1/2O2 —–CH3COCH3
Oxidation of Butanol- chemical engineers can as well produce acetone from a process of oxidation of Butanol. Rahman asserts, “The solvent used methanol, ethanol, propanol; n-butanol, ethylene glycol; propylene glycol, diethylene glycol, and triethylene glycol are useful solvents for hydrogenation reactions” (120). According to Arda et al., catalytic oxidation of butane by using cobalt and manganese acetate produces acetic acid under a temperature rate of 150-225 degrees Celsius, where butane undergoes oxidation to produce acetone of almost 75-80% yield (18).
Chemical formulae: CH3CH2CH2CH2CH3 + O2——-CH3COOH + CH3COCH
Oxidation of Isopropyl Benzene (Cumene) – Cumene is an organic compound produced from an integrated process of producing phenol. Production of phenol further involves, “alkylation of benzene with propene to obtain cumene, oxidation of cumene to cumene hydro peroxide, acid cleavage of cumene hydro peroxide to produce phenol and acetone” (Rahman 114). According to the explanation given by Arda et al. (21), cumene is simply a product obtained from synthesis of propylene and benzene and oxidation of the product to produce acetone and phenol.
Dehydrogenation of Isopropanol- Another important method of obtaining acetone is dehydrogenation of Isopropanol. Rahman postulates, “Isopropanol is widely regarded as an essential commodity in fine chemical synthesis which is used as a solvent in the industry and academia” (113).
Normally, hydrogenation of acetone leads to production of Isopropanol, and the vice versa is that dehydrogenation of Isopropanol produces acetone. The catalyst used in this process is Zinc Oxide (ZnO) (IARC 483). The following is the chemical formulae for dehydrogenation of Isopropanol.
Chemical formulae: (CH3)2CHOH ——(CH3)3CO + H2
Safety and environment concerns
It is important to consider personal health and environmental safety concerns while undertaking chemical production of acetone. Acetone is a chemical like any other and there are possibilities of causing harm to human beings, animals and the entire flora and fauna (Tremoulet et al. Para 6).
All chemicals used in the production of acetone comprise of some caution notices, which engineers of acetone should follow strictly to avoid explosive reactions. In special attention, engineers should ensure that they prevent contamination of soil, drains and surface water.
According to Arda et al. (17), engineers interested in the production of acetone should strictly adhere to all industrial principles governing the production and consumption of chemicals including handling and storing of hazardous chemicals.
Acetone forms explosive mixtures with air and is extremely explosive and users of this chemical should remain keen throughout. Engineers should address any accidental inhalation or suffocation and any accidental cases with any first aid before considering seeking health assistance from physicians.
Preliminary cost of the materials
For engineers to engage in the production of acetone, it is imperative to consider the preliminary cost of production. However, the cost of producing acetone depends entirely on the amount of acetone produced. The major aim of preferring to produce more attractive and affordable acetone in the market was to employ the use of isopropyl alcohol in producing acetone (Rahman 118).
The production of acetone using isopropyl alcohol must be in a position to demonstrate the aspect of saving. Several materials and chemicals were useful and mandatory for the successful production of acetone. In a bid to determine the overall production cost of acetone for this project, this study employed a program costing technique known as CAPCOST to estimate the price of equipment employed.
The following table provides a detailed cost data for the production of acetone of this study as adopted from an empirical study by Tremoulet et al.
Total Production cost for acetone using isopropyl alcohol
Summary of the literature review
Acetone is an organic compound produced using different chemical methods. However, this study dealt with the chemical production of acetone using isopropyl alcohol, which is an essential component in the production of acetone.
Acetone is one of the most widely used solvents in the world and can be used as intermediate for several commercial products such as acrylic plastic, bisphenol, polycarbonates and epoxy resin, paints and adhesives, etc. In other places, acetone has been useful in nurturing women beauty, where women have used acetone in removing nail cuticles (Tremoulet et al. Para 7).
Acetone is often a by-product of phenol, with scientists capable of producing acetone from a variety of materials including propylene, cumene, and isopropanol. Acetone production involves several chemical and physical processes including heating, vaporizing, reacting, cooling, condensing, flashing, heating and scrubbing.
Production of acetone begins in the feed drum and ends in the isopropyl column where acetone finally emerges. One chemical formula can be useful in summarizing the process of producing acetone. CH3-CHOH-CH3 CH3-CO-CH3 + H2 can summarize the chemical reaction process in the production of acetone.
It is also important to notice that production of acetone is a very reactive process that needs consideration of safety and environmental concern. All chemicals used in the production of acetone comprise of some caution notices, which engineers of acetone should follow strictly to avoid explosive reactions.
Acetone forms explosive mixtures with air and is extremely explosive and users of this chemical should remain keen throughout. Finally, engineers should address any accidental inhalation or suffocation and any accidental cases with any first aid before considering seeking health assistance from physicians.
Works Cited
Arda, Urun, Serkan Acarser, Muge Metin, Sila Gungor, and Ali Kucuk. Chemical Engineering Design Project: Acetone Production, 2009. Web.
IARC. Isopropyl Alcohol Manufacture by the Strong-Acid Process. Web.
Rahman, Ateeq. “Catalytic Hydrogenation of Acetone to Isopropanol: An Environmentally Benign Approach.” Bulletin of Chemical Reaction Engineering & Catalysis 5.2 (2010): 113 – 126. Print.
Tremoulet Mike, Mike Unton, and Ed Feng. Production of Acetone Using Catalytic Dehydrogenation of Isopropyl Alcohol, 1998. Web.