Introduction
Disorders that affect the immune system of the human body have a range of dangerous consequences since the organism becomes incapable of identifying the actual threats and properly managing them. Rheumatoid arthritis is a well-known example of an autoimmune disease even though its prevalence in the world is not extremely high compared to other health problems.
To understand the underlying causes of this disease and improve the existing pharmaceutical treatment options that slow its progression, it is pivotal to focus on its pathogenesis and thoroughly describe the existing disruptions in the work of different immune cells. This paper is aimed at describing the mechanisms of autoimmunity related to the development of rheumatoid arthritis concerning the promising treatment options.
Rheumatoid Arthritis and Its Development
Sex Biases in Rheumatoid Arthritis and Potential Causes
There are numerous physiological differences between males and females, and the dissimilarities often manifest themselves in the progression of diseases or atypical symptoms. The issue of sex biases in autoimmune disorders deserves special attention since it has a significant impact on the use of new risk prevention and treatment strategies. Autoimmune diseases include a range of common conditions that affect the health of people in the United States, and according to the statistics, being a female is an important risk factor in the majority of them (Rubtsova, Marrack & Rubtsov 2015). The amount of bias based on the biological sex of patients varies for different autoimmune diseases, but rheumatoid arthritis is not an exception.
The causes of sex bias in disorders that affect the proper work of the immune system are not fully studied, but modern researchers have a range of hypotheses with different degrees of credibility. According to Rubtsova, Marrack, and Rubtsov (2015), the immune responses of the female body are usually stronger than those of males. This fact explains obvious differences in the incidence of infectious diseases in men and women, but the reverse of the coin are the increased risks of autoimmune abnormalities that exist for females.
Nowadays, it is supposed that the factors that can explain differences between the sexes in autoimmune disorder prevalence rates include two X chromosomes instead of one and the fluctuations of hormones such as estrogen (Rubtsova, Marrack & Rubtsov 2015). Interestingly, even some socially conditioned differences such as dietary habits can be supposed to contribute to gender biases.
The Disease and the Stages of Development
The mechanisms of development of rheumatoid arthritis are extremely complicated since they involve a range of reactions that lead to the loss of tolerance to self-antigens. The disease typically manifests itself in joint inflammation associated with acute pain and general weakness, and it sometimes affects patients’ mental health (Shchetynsky et al. 2017). The development and progression of the disease concerning pathophysiological mechanisms and stages are thoroughly discussed in modern literature.
For instance, the study by Guo et al. (2018) singles out four stages of the disease that can take place in a fixed order or even occur simultaneously. The stages identified by the researchers refer to the processes of “triggering, maturation, targeting”, and the fulminant stage with long-term consequences, such as bone destruction (Guo et al. 2018, p. 1).
The existence of these stages is related to a common subtype of rheumatoid arthritis that is characterized by the presence of autoantibodies against proteins that have been modified due to citrullination (ACPA) (Guo et al. 2018). This type of rheumatoid arthritis has certain distinctive features related to the peculiarities of immune responses. This is why this factor’s role in the pathophysiology of the disease is emphasized.
Triggering and Maturation
The first stage of the disease is inextricably connected with ACPAs or IgM rheumatoid factors that appear in the body long before the manifestation of any symptoms. The appearance of ACPAs is regarded as a troubled “antibody response to a range of citrullinated proteins”, such as fibrin and histone proteins (Guo et al. 2018, p. 1).
The production of antibodies that are directed at proteins necessary for the proper functioning of the body is related to numerous external and internal influences such as inherited genetic risk factors and pernicious habits. Probably, it also has to deal with the consequences of severe infectious diseases (Li et al. 2013). The protein PTPN22 is also believed to contribute to the production of ACPAs through the inhibition of T cell activation (Guo et al. 2018). Therefore, the appearance of ACPAs has some potential reasons, some of which are underresearched.
The stage of maturation can take a lot of time, but the actual manifestation of unwanted symptoms does not exist yet. The production of self-antigens causes determinant spreading, and the influence of ACPAs leads to immune tolerance changes. Therefore, during this stage, citrullinated antigens manage to activate dependent T lymphocytes; the latter interact with B cells to increase the production of ACPAs (Guo et al. 2018). In their turn, the produced autoantibodies contribute to the negative long-term consequences of the disease.
Targeting and the Role of T and B Cells
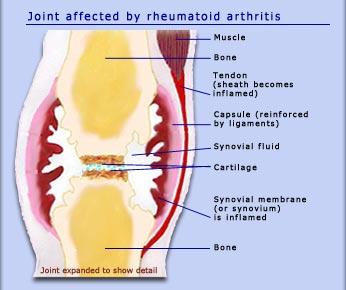
The stage of targeting involves the occurrence of the first physiological symptoms. During it, there is an inflammation of the tissues that line joints, the so-called synovial membrane (Figure 1). The activation of the immune system and the presence of ACPAs are associated with several negative consequences. The cells of the membrane, fibroblast-like synoviocytes, actively interact with the cells of the acquired immune system (B and T lymphocytes) to create an inflammatory cascade, and the immune system fails to reduce inflammation because of the activity of ACPAs.
Speaking about the stage in question, it is also pivotal to mention the role of white cells of the blood, monocytes. According to the findings made in the 1980s, they infiltrate the synovial membrane in large quantities, and their mal performance contributes to inflammation and even bone destruction (Puchner et al. 2018; Guo et al. 2018). Being activated by ACPAs, these cells start producing tumor necrosis factor-alpha, a cytokine that helps regulate different types of immune cells.
The role of autoreactive T cells in the disease being analyzed remains one of the key questions that interest modern researchers in the field. CD4 T cells are believed to play a pivotal role in the abnormal immune responses in patients with rheumatoid arthritis since they contribute to chronic synovitis of joints and the production of autoantibodies (Guo et al. 2018). Rheumatoid arthritis is often regarded as a disease mediated by T cells, and pathogenic or autoreactive T cells are believed to transfer it (VanderBorght et al. 2001).
Autoreactive T cells appear when the naïve forms of T cells leave the human thymus, start demonstrating new abilities after activation in the lymph nodes, and then migrate into the joints (VanderBorght et al. 2001; Mellado et al. 2015). In the diarthrosis, they undergo the process of cross-reactive activation initiated by antigens, and this interaction supposedly causes clonal expansion; at the same time, the process of clonal deletion that prevents autoimmunity proves abortive (Weyand, Yang & Goronzy 2014; VanderBorght et al. 2001). Having recognized autoantigens, some T cells react accordingly and secret various types of inflammatory cytokines, which contribute to the immune response.
B cells are also actively involved in the pathogenesis of the disease discussed in the given paper. As it has been stated, ACPAs can be found in the human body before the presentation of any external symptoms of rheumatoid arthritis. As is clear from some modern studies, the activity of autoreactive B lymphocytes is likely to be the key source of ACPAs (Pozsgay et al. 2016; Pelzek et al. 2017).
The phenomenon of B lymphocytes capable of ACPA production has not been fully studied yet, especially when it comes to the extent to which the levels of ACPAs predict the severity of external symptoms of the disease. According to Pelzek et al. (2017), B lymphocytes found in the synovial fluid of individuals with the ACPA-positive type of rheumatoid arthritis can demonstrate the spontaneous secretion of ACPAs. Despite several micro-level issues surrounding the onset of the disease, different types of immune cells do not lose the ability to cooperate, and T cells can contribute to the proliferation of B lymphocytes and the production of autoantibodies.
Inflammatory Mediators, Cytokines, and MMPs
The role of inflammatory mediators in the pathophysiology of the discussed disease cannot be overstated and should be analyzed concerning the phenomenon of signaling in the immune system. The members of the tumor necrosis factor superfamily such as TNF-alpha are listed among the key providers of signals that activate B lymphocytes and increase their chances to proliferate (McInnes & Schett 2007).
Interleukin 2 or IL-2 that belongs to the number of cytokines of the first type also fulfills an important function in the growth and expansion of T lymphocytes (Li & Rudensky 2016). The upregulation of accessory signals related to the activity of cells of the specific immune system is among the factors potentiating the immune response. Therefore, cytokines play a role in the promotion of immunogenicity vs tolerance.
The contribution of cytokines to the exacerbation of rheumatoid arthritis and its external symptoms should also be analyzed with special attention to the role that they play in the destruction of bones in the cases of RA. The abovementioned question is representative of the key research interests of scientists in the field of osteoimmunology that focuses on the interactions between the immune system and the internal skeleton. In rheumatoid arthritis, the interactions of various cells and cytokines upset the balance between processes related to the formation and resorption of the bones, leading to problems with osteoclast formation (Jung et al. 2014a).
In general, the role of cytokines in the destruction of bones within the affected joints is related to their ability to activate osteoclasts, specific bone cells responsible for the destruction of bone tissue. Speaking about the particular cytokines that participate in this process, it is necessary to mention interleukins 1 and 6 and TNF-alpha since these inflammatory cytokines stimulate the differentiation of osteoclasts, sometimes without the help of such protein as RANKL (Figure 2).
Matrix metalloproteinases, a range of endopeptidases that can destroy some collagens in the extracellular matrix, also contribute to bone destruction because they are required for the migration of osteoclasts. With that in mind, the suppression of some MMPs is regarded as a promising treatment for people with rheumatoid arthritis.
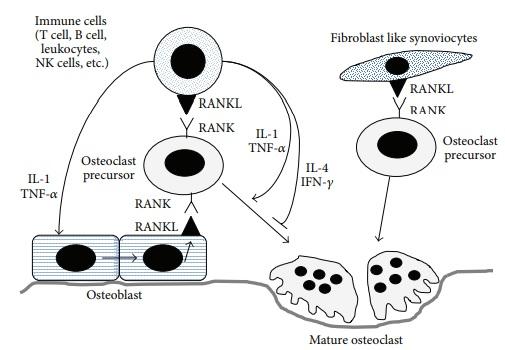
The idea that TNF-alpha mediates bone destruction finds extensive support in the existing literature. Activated T lymphocytes produce this cytokine, and its activity is strongly associated with the destruction of bones in the cases of cancer and inflammatory diseases (Jung et al. 2014a). The cytokine being discussed can contribute to these issues by impacting the production of M-CSF (a growth factor that regulates the functions of monocytes) and RANKL (Jung et al. 2014a).
This cytokine can also have an impact on the formation of osteoblasts. Interestingly, the use of pharmacological treatments aimed at blocking TNF-alpha is regarded as an effective option for patients with rheumatoid arthritis. For instance, the inhibitors of TNF-alpha used in the cases of RA help reduce inflammation and prevent the destruction of the bones, and these positive effects are also present in female patients with osteoporosis (Jung et al. 2014a). With that in mind, numerous treatment options for the disease are focused on TNF-alpha and its contributions to bone destruction.
Conclusion
To sum it up, rheumatoid arthritis is the disease that develops when the immune system fails to distinguish between dangerous and normal cells. The body provides an autoimmune response to its cells, which results in the deformation of joints, severe pain, and the brittleness of the bones. In the current literature, the mechanisms that let the body attack the tissues that do not pose a threat to physical health are described concerning many concepts, including defects in clonal deletion, the appearance of autoreactive T and B lymphocytes, and the dysregulation of cytokines.
Reference List
Guo, Q, Wang, Y, Xu, D, Nossent, J, Pavlos, NJ & Xu, J 2018, ‘Rheumatoid arthritis: pathological mechanisms and modern pharmacologic therapies’, Bone Research, vol. 6, pp. 1-14.
Joint affected by rheumatoid arthritis 2017. Web.
Jung, SM, Kim, KW, Yang, CW, Park, SH & Ju, JH 2014a, ‘Cytokine-mediated bone destruction in rheumatoid arthritis’, Journal of Immunology Research, vol. 2014. Web.
Jung, SM, Kim, KW, Yang, CW, Park, SH & Ju, JH 2014b, Osteoblast-derived RANKL binds to RANK on monocytes to differentiate them into mature osteoclasts. Web.
Li, MO & Rudensky, AY 2016, ‘T cell receptor signalling in the control of regulatory T cell differentiation and function’, Nature Reviews Immunology, vol. 16, no. 4, pp. 220-233.
Li, S, Yu, Y, Yue, Y, Zhang, Z & Su, K 2013, ‘Microbial infection and rheumatoid arthritis’, Journal of Clinical & Cellular Immunology, vol. 4, no. 6. Web.
McInnes, IB & Schett, G 2007, ‘Cytokines in the pathogenesis of rheumatoid arthritis’, Nature Reviews Immunology, vol. 7, no. 6, pp. 429-442.
Mellado, M, Martínez-Muñoz, L, Cascio, G, Lucas, P, Pablos, JL & Rodríguez-Frade, JM 2015, ‘T cell migration in rheumatoid arthritis’, Frontiers in Immunology, vol. 6. Web.
Pelzek, AJ, Grönwall, C, Rosenthal, P, Greenberg, JD, McGeachy, M, Moreland, L, Rigby, WFC & Silverman, GJ 2017, ‘Disease associated anti-citrullinated protein memory B cells in rheumatoid arthritis persist in clinical remission’, Arthritis Rheumatology, vol. 69, no. 6, pp. 1176-1186.
Pozsgay, J, Babos, F, Uray, K, Magyar, A, Gyulai, G, Kiss, É, Nagy, G, Rojkovich, B, Hudecz, F & Sármay, G 2016, ‘In vitro eradication of citrullinated protein specific B-lymphocytes of rheumatoid arthritis patients by targeted bifunctional nanoparticles’, Arthritis Research & Therapy, vol. 18, no. 1. Web.
Puchner, A, Saferding, V, Bonelli, M, Mikami, Y, Hofmann, M, Brunner, JS, Caldera, M, Goncalves-Alves, E, Binder, NB, Fischer, A, Simader, E, Steiner, CW, Leiss, H, Hayer, S, Niederreiter, B, Karonitsch, T, Koenders, MI, Podesser, BK, O’Shea, JJ, Menche, J, Smolen, JS, Redlich, K & Blüml, S 2018, ‘Non-classical monocytes as mediators of tissue destruction in arthritis’, Annals of the Rheumatic Diseases, vol. 77, no. 10, pp. 1490-1497.
Rubtsova, K, Marrack, P & Rubtsov, AV 2015, ‘Sexual dimorphism in autoimmunity’, The Journal of Clinical Investigation, vol. 125, no. 6, pp. 2187-2193.
Shchetynsky, K, Diaz-Gallo, LM, Folkersen, L, Hensvold, AH, Catrina, AI, Berg, L & Padyukov, L 2017, ‘Discovery of new candidate genes for rheumatoid arthritis through integration of genetic association data with expression pathway analysis’, Arthritis Research & Therapy, vol. 19, no. 1. Web.
VanderBorght, A, Geusens, P, Raus, J & Stinissen, P 2001, ‘The autoimmune pathogenesis of rheumatoid arthritis: role of autoreactive T cells and new immunotherapies’, Seminars in Arthritis and Rheumatism, vol. 31, no. 3, pp. 160-175. Web.
Weyand, CM, Yang, Z & Goronzy, JJ 2014, ‘T cell aging in rheumatoid arthritis’, Current Opinion in Rheumatology, vol. 26, no. 1, pp. 93-100.