According to Chang and Goldsby (2014), an atom is defined as, “the basic unit of an element that [participates in] chemical combinations” (p. 31). After many years of experimentation and development of theories by different scientists, it was established that an atom is made up of a positively-charged nucleus, which is surrounded by negatively-charged electrons. Moreover, different scientific theories postulated that an atom is a relatively small, indivisible particle that possesses a basic structure.
The structure of an atom includes a central, densely-packed nucleus, which is made up of smaller subatomic particles (the protons and neutrons), and it has electrons in the outer space or the “orbitals” (Chang & Goldsby, 2014; Siegfried, 2013). The current understanding of the composition of atoms and their structure has enabled scientists to develop insights into the configuration of different elements and molecules as well as the bonding behaviors and molecular polarity of various compounds (Zheng, 2012).
The history of scientific theories that explain about the composition of atoms and their structure can be traced back to the fifth century B. C. The earliest theory about atoms was made by Democritus (a Greek philosopher) who proposed that matter consisted of, “very small, indivisible particles, named atomos (meaning uncuttable or indivisible)” (Chang & Goldsby, 2014, p. 30).
However, the scientific evidence for Democritus’ claim was not available until the early 1800s when John Dalton provided a precise model of what is now known as an atom. This essay describes in greater detail the scientific theories of atoms and their structure, beginning with Dalton’s theory to the current models. In the subsequent discussions, it will become clear that there is no single scientific theory, which is made up of pure facts (Bronowski, 2011).
Instead, through scientific thinking and reasoning, scientists are capable of questioning the proposed theories before refining them in a way that brings out the truth in “hidden likenesses” (Bronowski, 2011). Moreover, through scientific theories, scientists have gained an upper hand in creating order in nature’s superficial appearances.
The Development of the Atomic Theory
Dalton’s Theory
John Dalton was a scientist and schoolteacher who was the first person after Democritus to describe the chemistry behind atomism. In 1808, Dalton proposed a more precise model of an atom whereby he noted that it was a small unit of matter that could not be divided into smaller parts (Chang & Goldsby, 2014). In summary, Dalton’s theory held that an atom is the basic building block of an element.
Moreover, in any given element, Dalton noted that there were identical atoms, which had similar sizes, weight, and chemical characteristics. However, since different elements had different atoms, it was possible for the atoms of two elements to come together to form compounds (figure 1).
Nevertheless, Dalton noted that when the atoms of different elements came together to form compounds, there was no possibility that the atoms could undergo creation or destruction, but they could be separated, combined, or rearranged through a chemical reaction (Chang & Goldsby, 2014; Rocke, 2005).
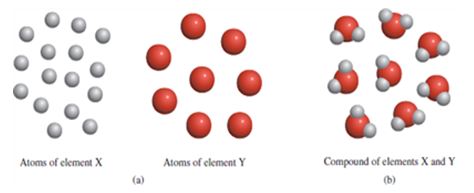
In essence, Dalton’s atomic theory of matter supported Democritus’ concept that an atom could not be divided or “cut”, but it was much more detailed than the latter (Chang & Goldsby, 2014). However, from the foregoing, it is worth-noting that Dalton’s theory made no reference to the composition of atoms let alone their structure.
Despite lack of knowledge and understanding regarding the composition and the structure of atoms, Dalton noted that hydrogen atoms were different from oxygen atoms because they had dissimilar chemical properties.
Dalton’s theory was very important because it provided scientific support for the proposals made in the law of definite proportions. Additionally, the theory supported the law of multiple proportions as well as the law of conservation of mass due to its assertion that the atoms, which make up elements, cannot be created or destroyed during a chemical reaction (Rocke, 2005).
Thompson’s Atomic Model
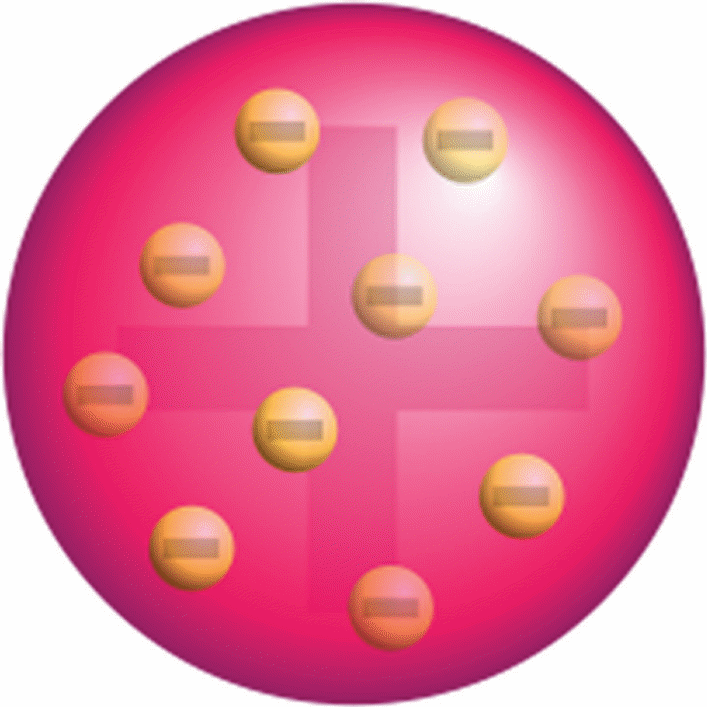
The first attempt to describe the internal structure of an atom was made by Thompson in 1897 (Zheng, 2012). In his theory, Thompson proposed that the structure of an atom comprised a positively-charged sphere that was surrounded by negatively-charged electrons (refer to figure 2). More specifically, Thompson’s structure was known as the “plum-pudding” model because it was similar to a cake that contained raisins spread out randomly (Siegfried, 2013).
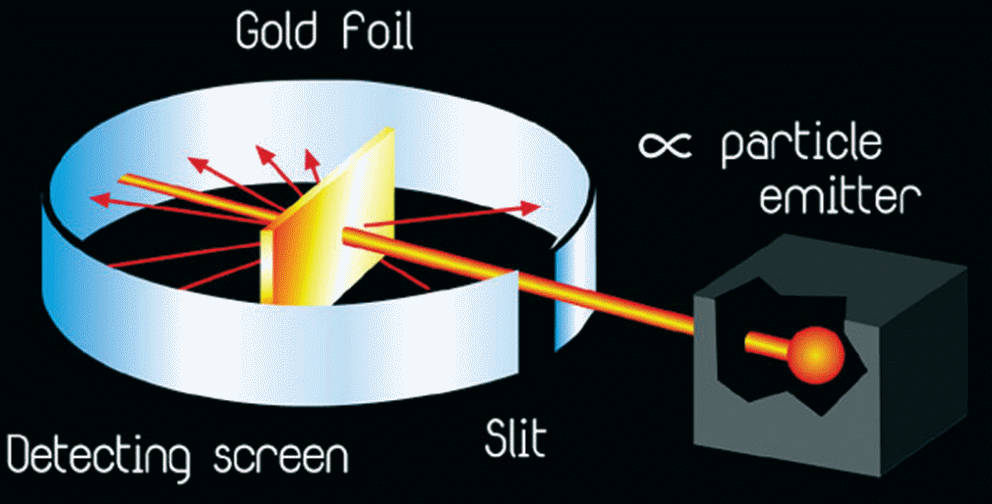
Based on this theory, Thompson took credit for discovering that an atom possessed negatively-charged particles known as electrons, which could deflect cathode rays (Zheng, 2012). Nevertheless, Thompson’s concept, particularly the deflection of cathode rays was disproved by Ernest Rutherford’s experiment in which he used alpha-particles and thin gold foils to test the deflective effect of electrons as proposed earlier (refer to figure 3).
The findings of this experiment revealed that only a small fraction of alpha-particles was deflected by the gold atoms as opposed to Thompson’s assertion that all or none of the particles would have been deflected (Zheng, 2012).
From this experiment, Rutherford proposed that an atom possesses a small centrally-placed mass of positive charge that was responsible for the sharp deflection of a fraction of the alpha-particles, which came into contact with it (Kragh, 2012). Accordingly, Rutherford’s model showed that an atom consisted of a small, dense mass of positive charge, which was called the nucleus and an empty outer space that housed the electrons (Zheng, 2012).
Bohr’s Theory
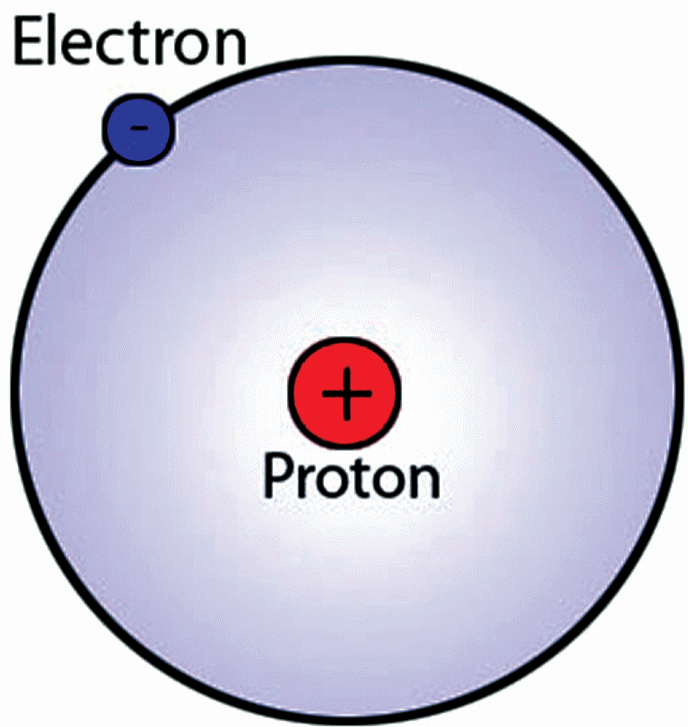
Although the aforementioned atomic theory by Rutherford was accurate in that it predicted the existence of a dense nucleus in the core of an atom, it still had a few shortcomings. For example, Rutherford proposed that the negatively-charged electrons spiraled around the dense positively-charged core. This was wrong because the laws of physics show that positive charge attracts negative charge and vice versa (Siegfried, 2013).
Therefore, if there were negative electrons in an atom, they would have been attracted to the dense positive core instead of moving around it. This led other scientists to propose that the only way that the negatively-charged electrons could be housed in an atom that contained a positively-charged nucleus was if the electrons moved around the nucleus in the same way that the planets revolve around the sun (Zheng, 2012).
This proposal was countered by Niels Bohr in his theory, which predicted that energy can be quantized. Therefore, instead of focusing on the laws of classical physics, Bohr approached the discussion on the movement of electrons around the nucleus from the perspective of quantum physics.
Accordingly, Bohr noted that the transfer of energy involved a minimum amount or a “quantum”, which is also equivalent to the energy contained in one photon. Furthermore, Bohr challenged the then existing classical laws because he believed that they did not provide sufficient evidence to explain the nature of electron movement around the nucleus.
More specifically, Bohr argued that if the classical laws were to hold, then the movement of electrons in an atom could be restricted because the electrons were in constant collision with the nucleus (Zheng, 2012). However, based on the concept of quantum physics, Bohr’s atomic theory predicted that the movement of electrons around the nucleus occurred at specific energy levels.
This means that an electron must possess a certain amount of energy to exist in a specific energy level. If an electron cannot fit in any level, then it must radiate energy until it achieves the threshold for entering a specific energy level (figure 4). Nonetheless, Bohr’s model served to explain the behavior of simple atoms such as the hydrogen atom, and failed to provide evidence for the movement of electrons within complex atoms (Siegfried, 2013; Zheng, 2012).
Modern Atomic Theories
The most current atomic models had been proposed by different scientists including Erwin Schrodinger and Werner Heisenberg. Schrodinger’s theory borrowed from the concept of the duality of matter, which had been proposed by Louis de Broglie. Schrodinger then improved Bohr’s model by predicting that the movement of electrons around the nucleus could be explained using an equation that equated electrons to waves (Zheng, 2012).
Therefore, using Schrodinger’s equation, it was possible to calculate the most probable location of an electron in the space around the nucleus. Here, note that Schrodinger’s theory differed with Bohr’s model in that it did not predict that an electron could be found in a specific location at any given time. Instead, Schrodinger proposed that there are many uncertainties surrounding the specific location of electrons in an atom.
Subsequently, Heisenberg concurred with Schrodinger’s observations by proposing that due to the duality of matter, it was not possible to determine the momentum and specific location of an electron at any given moment (Zheng, 2012). As a result, Heisenberg concluded that the momentum and location of electrons in an atom can only be determined using probability functions (refer to figure 5).
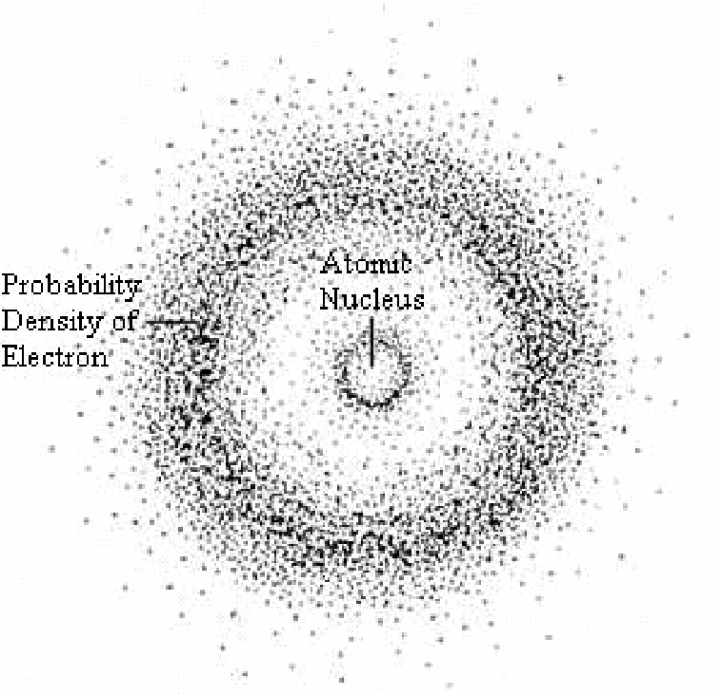
Conclusion
An atom refers to the smallest unit of an element, which participates in chemical reactions. During a chemical reaction, atoms cannot be created or destroyed, but instead, they can be combined, rearranged, and separated. The process of determining the properties and internal structure of an atom through the development of scientific theories can be traced back to the early nineteenth century.
Dalton’s theory is one of the well-known atomic theories that formed the basis for the development of the current understanding of atoms and their structure. Other important scientists that were involved in the development of atomic theories are Thompson, Rutherford, Bohr, and Schrodinger.
From the foregoing discussions, it appears that scientific theories are not a group of facts, but they are very important assumptions that guide scientists in the development of more refined explanations regarding the nature and existence of different things in the world.
References
Bronowski, J. (2011). The nature of scientific reasoning. In L. H. Peterson, J. Bizup, A. E. Fernald & M. Goldthwaite (Eds.), The Norton reader: An anthology of nonfiction (pp. 935-938). New York, NY: W. W. Norton & Company.
Chang, R., & Goldsby, K. A. (2014). General chemistry: The essential concepts (7th ed.). New York, NY: McGraw-Hill Education.
Kragh, H. (2012). Rutherford, radioactivity, and the atomic nucleus. Web.
Rocke, A. J. (2005). In search of El Dorado: John Dalton and the origins of the atomic theory. Social Research, 72(1), 125-158.
Siegfried, T. (2013). When the atom went quantum: Bohr’s revolutionary atomic theory turns 100. Science News. Web.
Zheng, A. (2012). The evolution of atomic theory. Young Scientists Journal, 1(12), 74 76. Web.