Introduction
Evolution has a tendency to encourage the advancement of a single favorable female and male phenotype for each species (Brazill-Boast, Griffith, & Pryke 2013). The incidence of more than one discrete, heritably controlled colour form in a single population is referred to as genetic colour polymorphism (Gilby, Pryke, & Griffith 2009). This phenomenon occurs in birds, fish, insects, frogs, and lizards (Dijkstra et al. 2009). Organisms displaying these variations are termed as morphs. Apart from appearance, discrete morphs can also show variation in features such as behaviour and bodily processes. According to Gilby, Pryke and Griffith, the examination of these species reveals detailed information on a number of fundamental ecological and evolutionary procedures (2009). A complete understanding of these processes plays a significant role in the comprehension of speciation and diversity. For instance, it is assumed that equilibrium between diverse genetic morphs is displayed by the subsistence of a stable genetic polymorphism in a distinct interbreeding populace. This presents an evolutionary challenge that can only be resolved by a study that combines ecological, behavioural and genetic processes (Gilby, Pryke, & Griffith 2009).
Polymorphism confers several evolutionary adaptations to severe situations and varying habitats and nooks. This is evident in “the existence of ratio-clines whereby the frequencies of morphs varies spatially across different environments” (Gilby, Pryke, & Griffith 2009, p.222). Polymorphism encourages the use of assorted environmental resources and ensures constancy of the population (Forsman et al. 2008). Disruptive selection and ecological factors are suggested as the key causes of polymorphisms in birds where selection gives preferentiality to the extreme phenotype of an attribute that is otherwise normally dispersed (Pryke 2007). For example, some birds take advantage of colour polymorphism (plumage colouration) for efficient hunting or protective camouflage. In such cases, the conspicuous colour usually matches the birds’ habitat such that it becomes hard for their prey or predator to distinguish them. Access to mates also benefits from polymorphisms in the behavior of male species. For example, highly aggressive males usually compete against their non-territorial morphs for mates.
In lizards (Lacerta vivipara), the females show a polymorphism in ventral colour, which is related to differences in reproduction, distribution, as well as vulnerability to competition for resources (Vercken 2008). Female contest competitions promote the evolution of signals associated with the ability of females to compete. The choice of male mate and other striking features also identify suitable females during mating competitions. Lizards have red, orange or mixed colouration on their ventral sides, but recent studies reveal that these colour morphs are not distinct and can be further distinguished by spectrophotometry (Vercken, Sinervo, & Clobert 2008). Lizards with different colours show varying sensitivities to intrasexual competition and to morph-specific competition (Vercken 2008). The reproductive victory of the different morphs relies on the rate of recurrence of each color morph in the environment (Vercken, Sinervo, & Clobert 2012).
Color designs in fish are frequently multicomponent indicators that can be employed in communication within species (Price et al. 2008). Intra and inter-sexual connections also benefit from these signals (Maan & Sefc 2013). Selective demands compelled by competitors, potential mates, marauders, and prey influence the colour patterns in fish. This paper aims at investigating the relationship between colour and aggression using the red devil cichlid fish (Amphilophus labiatus). It is hypothesised that the cichlids of gold morph is more aggressive than dark morphs.
Method
Conditions and materials
Wild red devil cichlids were caught from the Hazelwood Lake in Gippsland, Victoria and brought to the Jock Marshall Reserve in Monash University, Clayton campus. They were randomly allocated between two 2500L flume tanks housed in a 26˚C indoor facility, which ran a 12-hour light/12-hour dark cycle. These tanks were labelled Population A and Population B, each consisting of approximately 50% gold morphs and 50% dark morphs of cichlids.
Three 200L glass tanks were used to observe fish behaviour. These were filled with water from the flume tanks and kept at 26˚C. The sides of the glass tanks were covered, and each tank was fitted in the middle with a transparent divider and removable opaque barriers (Figure 1).
Procedure
Three contest types based on the colour morphs of red devil cichlids were examined with 11 pairings allocated in each, in which gold morphs were paired with gold morphs (type 1); gold morphs were paired with dark morphs (type 2), and dark morphs were paired with dark morphs (type 3). A total of 66 cichlids were observed.
One fish from each population was placed in the tank either side of the dividers, and was left to acclimatise to the change of environment in isolation for 10 minutes. Cichlids were taken from separate tanks to assure they were not familiar with one another, which was thought to increase the number of aggressive behaviour. Only fish within 2.0 cm of each other were contested against the other. After acclimatisation, the opaque barrier was removed, and behaviour was observed from above for another 10 minutes.
Six types of behaviour were defined:
- Approach – approaching the barrier in a non-aggressive manner;
- Fleeing or retreating – swimming away from the other fish;
- Submissive behaviour – fin tucking and a higher position in the tank;
- Contest – gill and fin flaring and a lower, dominant position;
- Charging – rapidly and aggressively swimming against the barrier; and
- Charging with attempted mouth-lock – charging and attempting to bite other fish.
These were tallied in a prepared data sheet, in which a new behaviour count was recorded after a fish disengages. Time to first behaviour and which fish it was initiated by were also recorded, as well as both their final positions in the water at the end of the trial. After each trial, both cichlids were placed in a separate flume tank from Population A and B to avoid being retested.
Data collected were analysed using the IBM SPSS Statistics 20 program. Analysis tests performed were independent sample t-tests, Mann-Whitney U-tests, a Kruskal-Wallis test, and a Spearman correlation.
Results
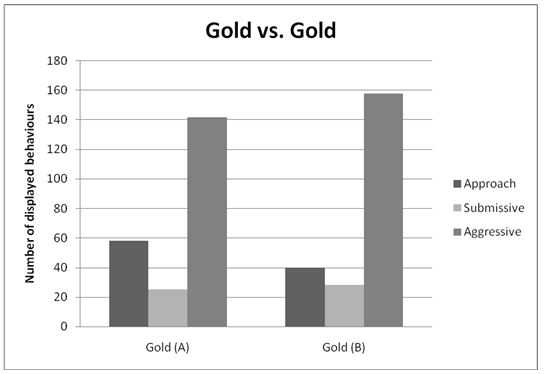
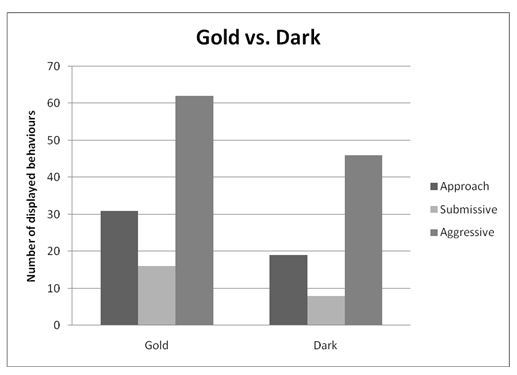
The six behaviours recorded were condensed and simplified into three general behaviours during analysis. These were Approach, Submissive (made up of Fleeing/retreating and submissive behaviour), and Aggressive (made up of Contest, Charging, and Charging with bite). Figures 1, 2, and 3 illustrate the totals of these behaviours for contest type 1, 2 and 3 respectively.
Gold versus Dark
Overall aggressive behaviour in gold cichlids was compared against dark cichlids (Figure 4). To determine the relationship between colour morph and aggression, a Mann-Whitney U-test (with α =.05) was used as the Shapiro-Wilk test of normality and Levene’s test of homogeneity of variance were violated. The Mann-Whitney U-test revealed that cichlids of gold morph were more aggressive (Mean Rank = 36.30, n = 33) than those of dark morph (Mean Rank = 30.70, n = 33). However, this was not statistically significant, U = 452.00, p =.229, two-tailed (Table 1).
Contest Type
Aggression was compared for the different contest types observed (Figure 5). After ranking the original aggressive behaviour, a Kruskal-Wallis test (with α =.05) was used to evaluate the effects of contest type on aggression as normality and homogeneity of variance could not be assumed. The Kruskal-Wallis test revealed aggressive behaviour varied statistically significantly across gold vs. gold (Mean Rank = 41.18, n = 22), gold vs. dark (Mean Rank = 25.48, n = 22), and dark vs. dark (Mean Rank = 33.84, n = 22), χ2(2, N = 66) = 7.584, p =.023 (Table 2).
Contest Type 1: Gold versus Gold
An independent-samples t-test (with α =.05) was used to compare the number of aggressive behaviour in gold cichlids of population A (n = 11) against gold cichlids of population B (n = 11). Cichlids of population A (M = 12.91, SD = 10.124) showed an average of 1.455 aggressive behaviour less than those of population B (M = 14.36, SD = 17.391). However, this was not statistically significant (t (20) = -2.40, p =.813, two-tailed).
Contest Type 2: Gold versus Dark
Because the assumption of normality was violated, a Mann-Whitney U-test (with α =.05) was used to evaluate how aggression varied in gold cichlids and dark cichlids when contested against each other. The test revealed no statistical significance between aggressive behaviour in gold (Mean Rank = 11.77, n = 11) and dark (Mean Rank = 11.23, n = 11) cichlids, U = 57.5, p =.847, two-tailed.
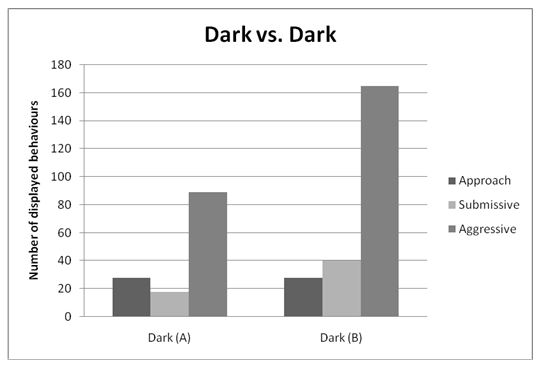
Contest Type 3: Dark versus Dark
A Mann-Whitney U-test (with α =.05) was used to determine a difference in aggression between dark cichlids of population A (n = 11) and dark cichlids of population B (n = 11). Cichlids of population B showed more aggressive behaviour (Mean Rank = 12.55) than those of population A (Mean Rank = 10.45). However, this was not statistically significant, U = 49.00, p =.478, two-tailed.
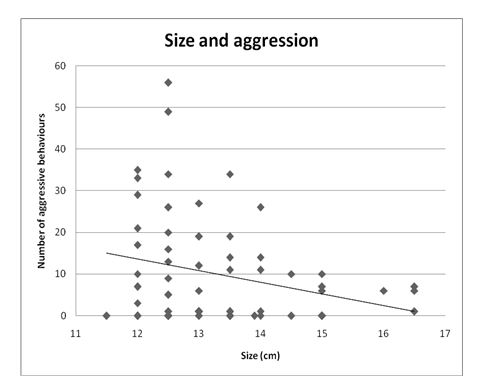
Size and Aggression
Because all sizes except 12.0cm, 14.0cm, and 15.0cm violated assumptions of normality, a Spearman’s correlation coefficient (with α =.05) was calculated to examine the relationship between size and aggression in red devil cichlids. A weak, negative relationship between size and number of aggressive behaviour displayed was found, rs = -.225. However, this was not statistically significant, p =.07, two-tailed, N = 66 (Figure 6).
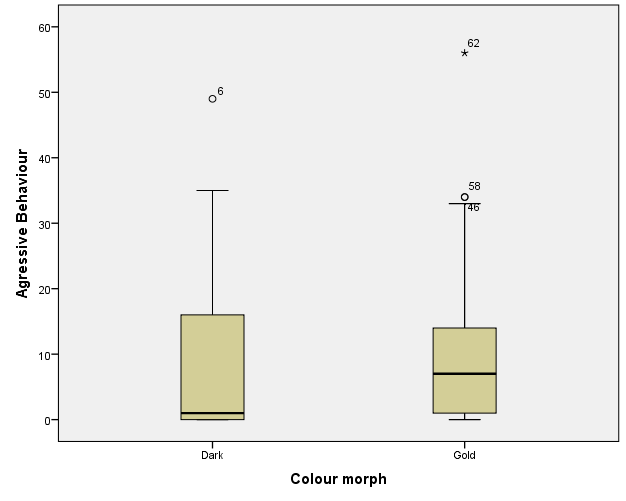
Table 1: Mann-Whitney U-test for aggressive behaviour in gold and dark red devil cichlids when contested together
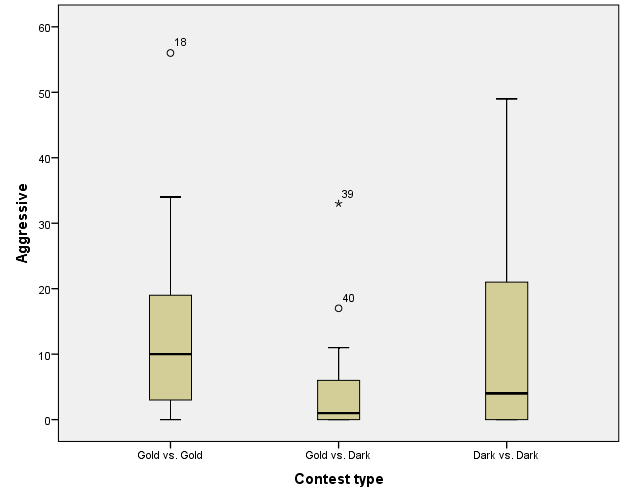
Table 2: Kruskal-Wallis test for effects of contest types: gold vs. gold, gold vs. dark, and dark vs. dark on aggressive behaviour
Discussion
Approach
The highest level of approach behaviour was realized when similar morphs were allowed to contest meaning that placing similar morphs in the tank led to peaceful coexistence since they did not see one another as threats.
Submissive
The highest level of submissiveness was observed when dark cichlids were contested with dark cichlids. The fish fled or retreated from each other and tucked their fin. They also occupied a higher position in the tank implying that it was the most comfortable position that the fish were accustomed to in their natural habitat. However, least submissiveness was observed when dark cichlids were contested with gold cichlids. That implied that when separate colour morphs were brought together there were high tendencies of aggression in an attempt to establish respective positions in the dominance hierarchy. The more dominant morphs sought to assert their position in the habitat hence high aggression.
Aggression
The aggression level was highest when gold cichlids were contested against gold cichlids. When gold versus dark cichlids were contested it was seen that the level of aggression was higher in the gold cichlids than in the dark ones. There was also high aggressive behaviour when dark cichlids were contested against their dark counterparts. The Mann-Whitney U-test revealed that cichlids of gold morph were more aggressive than those of dark morph. However, that aggressiveness was not statistically significant suggesting that the gold colour repressed attack probably because it inspired fear responses (Barlow & Wallach 1976).
Aggression by Contest Type
The results revealed that aggressive behaviour varied across all contest types (the variation was statistically significant). Contest type 1 (gold versus gold cichlids of population A) showed less aggressive behaviour less than population B. However, the difference was not statistically significant because the two populations (A and B) belonged to the same position in the dominance hierarchy and could not, therefore, display more aggressiveness towards each other. The test revealed no statistical significance between aggressive behaviour in gold and dark cichlid. The observations followed Barlow’s observation that gold dominance was only distinct in groups during the establishment of inter-individual relationships (1976).
Size and Aggression
Normally, cichlids are highly aggressive fish (Barlow 2000). It is thought that this aggressiveness can be minimized by placing similar sized fish in identical tanks as well as ensuring that resources such as food and space are abundant (Cichlid fish n.d.). There was a weak negative correlation between size and number of aggressive behaviour displayed. It was seen that the smaller cichlids showed higher aggression compared to their larger counterparts. However, the relationship between size and aggressiveness was not statistically significant. That observation agreed with a separate study by Oldfield (2011, p.340) that fish size did not play a significant role in the aggressiveness of cichlids. A separate study by also showed that cichlids’ aggressiveness (Neolamprologus pulcher) was not determined by body size (Reddon 2013).
Numerous studies in human and animal models depicted that there was an evolutionary-derived association of the colour red with dominance, communication and sexual selection (Roberts 2010). The results of the investigation were consistent with an observation by Reebs that aggression enabled fish to organize their comparative positions in a dominance hierarchy (2008). Therefore, when eleven members from the gold morph were placed in tanks with an equal number of their less aggressive counterparts (dark morphs) it was seen that the level of aggressiveness increased thereby developing a pecking order that placed each member in its position in the hierarchy. It could be concluded that the experimental results supported the experimental hypothesis that the cichlids of gold morph were more aggressive than dark morphs.
References
Barlow, G. W 2000, The cichlid fishes: nature’s grand experiment in evolution, Perseus Books Group, Massachusetts.
Barlow, G. W., & Wallach, S. J 1976, “Colour and levels of aggression in the Midas cichlid,” Animal Behavior, vol.24 no.4, pp. 814-817.
Brazill-Boast, J., Griffith, S. C., & Pryke, S. R 2013, “Morph-dependent acquisition and fitness in a polymorphic bird,” Evolutionary Ecology, vol.27 no.3, pp.469-475.
Cichlid fish n.d. Web.
Dijkstra, P. D., Dijk, S., Groothuis, T. G. G., Pierotti, M. E. R., & Seehausen, O 2009, “Behavioral dominance between female color morphs of a Lake Victoria cichlid fish,” Behavioral Ecology, vol. no. pp. 1-8.
Forsman, A., Ahnesjö, J., Caesar, S., & Karlsson, M 2008 “A model of ecological and evolutionary consequences of color polymorphism,” Ecology, vol.89 no.1, pp.34-40.
Gilby, A. J., Pryke, S. R., & Griffith, S. C 2009, “The historical frequency of head-colour morphs in the Gouldian Finch (Erythrura gouldiae),” Emu, vol.2009 no.109, pp. 222–229.
Maan, M. E. & Sefc, K. M 2013, “Colour variation in cichlid fish: developmental mechanisms, selective pressures and evolutionary consequences,” Seminars in Cell & Developmental Biology, vol.24 no.2013, pp.516-528.
Oldfield, R. G. 2011, “Aggression and welfare in a common aquarium fish, the Midas cichlid,” Journal of Applied Animal Welfare Science, vol.14 no. 2011, pp. 340–360.
Price, A. C., Weadick, C. J., Shim, J., & Rodd, F. H 2008, “Pigments, patterns, and fish behavior,” Zebrafish, vol.5 no.4, pp. 297-307.
Pryke, S. R 2007, “Fiery red heads: female dominance among head color morphs in the Gouldian finch,” Behavioral Ecology, vol.2007 no. 18, pp. 621-627.
Reddon, A. R 2013, Social decision-making in a group living cichlid fish. Web.
Reebs, S. G 2008, Aggression in fishes. Web.
Roberts, S. C 2010, “Distinguishing between perceiver and wearer effects in clothing color-associated attributions,” Evolutionary Psychology, vol.8 no.3, pp. 350-364.
Vercken, E 2008, “Ventral colour polymorphism correlates with alternative behavioural patterns in female common lizards (Lacerta vivipara),” Écoscience, vol. 15 no.3, pp.320-326.
Vercken, E., Sinervo, B., & Clobert, J 2008, “Colour variation in female common lizards: why we should speak of morphs, a reply to Cote et al.,” Journal of Evolutionary Biology, vol.21 no.2008, pp.1160–1164.
Vercken, E., Sinervo, B., & Clobert, J 2012, “The importance of a good neighborhood: dispersal decisions in juvenile common lizards are based on social environment,” Behavioral Ecology, vol.2 no.2012, pp.1-9.