Objective
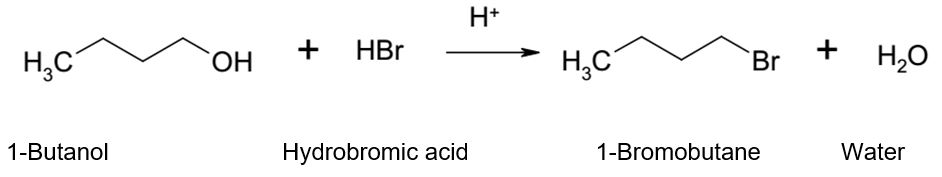
The goal of this experiment was to synthesize 1-bromobutane from 1-butanol via an SN2 reaction mechanism and confirm the presence and identity of the resultant primary alkyl halide using infrared (IR) spectroscopy.
Introduction
A substitution reaction is a form of chemical reaction where a functional group or an atom is substituted with a different functional group or atom. Substitution reactions fall into two main categories namely substitution nucleophilic unimolecular (SN1) and substitution nucleophilic bimolecular (SN2) reactions. The SN2 substitution reaction occurs when the attacking atom or group is a strong nucleophile that hits the electrophile from the rear end thereby ousting the leaving group. SN2 reactions also occur in polar aprotic solvents.
The ease of occurrence of SN2 reactions depends on the conformation of the alcohol. Overall, SN2 reactions occur with more ease in methyl alcohol than in secondary and tertiary alcohols because there is a less steric hindrance that enables the nucleophile to ‘attack’ the substrate from the rear end. SN2 reactions are one-step concerted reactions that do not have intermediates. In contrast, an SN1 reaction happens when the offensive group is a weak nucleophile or in the presence of a polar protic solvent.
The progress of SN1 reactions involves a time-consuming rate-limiting stage where a carbocation is formed. The carbocation intermediate requires utmost stability. Therefore, SN1 reactions occur readily in tertiary alcohols.
Alcohols react with the strongly acidic hydrogen halides via substitution reactions to produce water and an alkyl halide. However, alcohols are not good leaving groups due to the presence of the hydroxyl group (OH), which is a strong base. Therefore, an acid is needed to catalyze the reaction of alcohols with hydrogen halides.
The role of the acid is to protonate the alcohol hydroxyl group and enhance its leaving capabilities. A good leaving group can be described as a group that holds on to the pair of electrons from the carbon-oxygen bond in the course of dislodgment by a nucleophile. In most cases, a good leaving group is a steady species that is also a weak base. Other strong Lewis acids can also be used in the place of hydrohalic acids such as hydrochloric acid or hydrobromic acid. However, bromide and iodide ions form stronger nucleophiles than chloride ions.
Therefore, hydrogen chloride cannot react with primary or secondary alcohols except if zinc chloride or a related Lewis acid is included in the reaction mixture. Zinc chloride produces a multiplex with the alcohol as a result of the connection with the oxygen atom through a pair of free electrons on the oxygen atom. The complex improves the hydroxyl’s leaving group capability adequately for it to be displaced by the chloride ion.
Materials and Methods
1-butanol
State: colorless liquid
Chemical formula: C4H10O
Molar Mass: 74.12 g/mol
Boiling point: 117.6 °C
Density: 0.81 g/mL at 25 ° C
Hazards: Highly flammable, toxic, and irritates the skin and mucous membranes.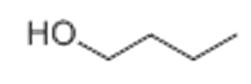
Hydrobromic acid
State: colorless liquid
Chemical formula: HBr
Molar mass: 80.91 g/mol
Boiling point: -67 °C
Density: 1.49 g/mL at 25 °C
Hazards: Irritant and corrosive.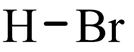
Sulfuric acid
State: clear, colorless oily liquid
Chemical formula: H2SO4
Molar mass: 98.08 g/mol
Boiling point: 290 °C
Density: 1.840 g/mL at 25 °C
Hazards: Flammable, toxic, corrosive, and irritant.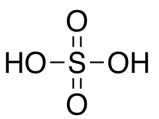
Sodium bicarbonat
State: White solid
Chemical formula: NaHCO3
Molar mass: 84.01 g/mol
Boiling point: 851 °C
Melting point: 300 °C
Density: 2.16 g/mL at 25 °C
Hazards: toxic when ingested or inhaled, irritant.
Sodium sulfate
State: White solid
Chemical formula: Na2SO4
Molar mass: 142.04 g/mol
Melting point: 884 °C
Density: 2.68 g/mL at 25 °C
Hazards: Highly flammable.
1-bromobutane
State: colorless to pale yellow liquid
Chemical formula: C4H9Br
Molar mass: 137.02 g/mol
Boiling point: 100-104 °C
Density: 1.276 g/mL at 25 °C
Hazards: Flammable, irritant.
Procedure
6.2 ml of 1- butanol was measured and put in a 100 ml round bottom flask using a measuring cylinder. 10 ml of 48% of hydrobromic acid (HBr) was added to the flask with careful whirling after which 4 ml of concentrated sulfuric acid (H2SO4) was added gradually and cautiously into the flask. The contents were swirled constantly, which caused the flask to become hot. The hot flask was cooled in an ice bath followed by the addition of a piece of boiling chip. The flask was connected to a reflux condenser and a sink aspirator and heated at reflux for 45 minutes.
After heating, the flask together with its contents was cooled to cool to room temperature. 10 ml of de-ionized water was then added to the flask through the condenser. A new boiling chip was added, and the apparatus was changed to a simple distillation system by detaching the vacuum. The flask contents were distilled into a 25 ml round bottom flask dipped in an ice bath. The distillation process came to an end when the temperature of the distillate reached 100oC. Thereafter, the aqueous phase was removed using a pipette. The product was kept for IR spectroscopy.
The organic layer was washed once using 5 ml of de-ionized water followed by two washes with 5 ml of 5 % sodium bicarbonate and ultimately with 5 ml of deionized water. The organic phase was separated from the aqueous phase and kept in a waste flask. The organic layer was then dried over anhydrous sodium sulfate and transferred to a pre-weighed sample bottle. The weight of the product was obtained and used to calculate the percentage yield. The identity of the products formed was established by examining the products using IR spectroscopy.
Results
Theoretical yield
Mass of 1-butanol = 0.81 g/mL * 6.2 mL = 5.022 g
Moles of 1-butanol = 5.022 g / 74.12 g/mol = 0.0677 mol
Theoretical yield = (0.0677 mol *137.02 g/mol) / 1 mol of 1-bromobutane = 9.28 g
Percent yield
Empty sample bottle = 14.97 g
Sample bottle+ product = 15.48 g
Mass of the product = 15.48 g – 14.97 g = 0.51 g
Percent yield = (Actual yield/ theoretical yield) * 100 = (0.51 g/9.28 g)*100= 5.49 %
Synopsis of the results
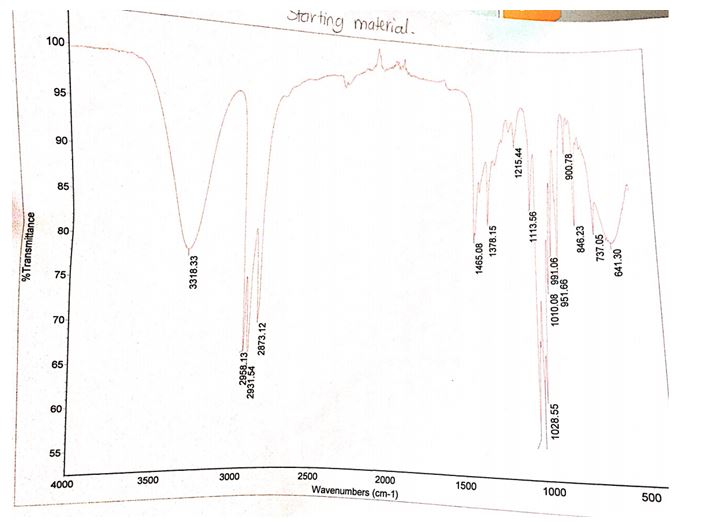
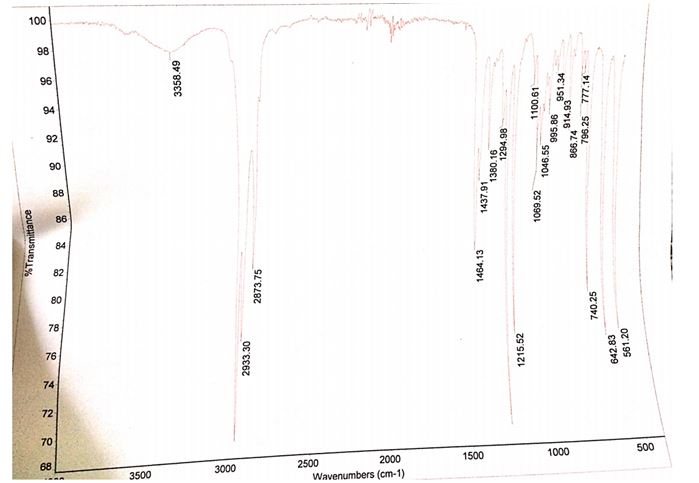
The product that was obtained from the experiment weighed 0.51 g while the computed theoretical yield was 9.28 g. Therefore, the percentage yield of bromobutane was 5.49%. The IR spectrum of the starting material contains a peak between 3300 and 3500 cm-1 while the IR spectrum of the product does not have this peak at the same wavelengths.
Another difference between the two spectra is the presence of a stretch band at approximately 700 cm-1 in the product and the absence of the same stretch band in the starting products.
Discussion
The primary 1-butanol was converted into 1-bromobutane by reacting with hydrobromic acid. The bromide ion acted as a nucleophile, which donated a pair of electrons to the electrophile. A small quantity of concentrated sulfuric acid (H2SO4) was used in the experiment as a catalyst to protonate the hydroxyl group to form a good leaving group.
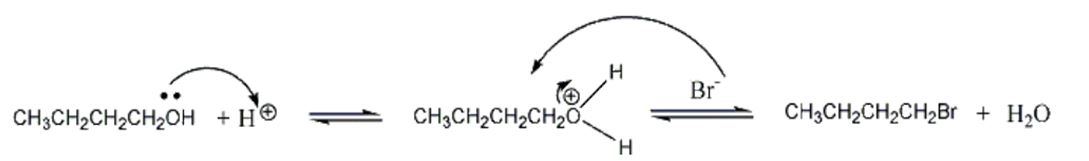
Protonation of the oxygen in the hydroxyl group allowed it to donate a pair of electrons to the hydrogen ion to generate a new oxygen-hydrogen bond in a process that converted the alcohol into an oxonium species (-OH2+), which was a good leaving group. The bromide ion then attacked the electrophile from the rear in an intensive one-step process causing the leaving group to exit from the front leading to the conversion of 1-butanol to 1-bromobutane.
The identity of the product obtained was established by relating the IR spectrum of the starting material to the spectrum of the final product. The presence and absence of functional groups unique to the reactants and products were ascertained by checking for the presence of the O-H stretching bond as well as the C-Br bonds in the IR spectra of the reactants and products. The spectrum of the starting material (1-butanol) showed a broad peak between the wavelengths of 3300 and 3500 cm-1, which was consistent with the absorbing region of the O-H bond.
This peak was nonexistent in the 1-bromobutane spectrum signifying the lack of the OH group in the product. Moreover, the incidence of a C-Br stretch band at roughly 700 cm-1 in the IR spectrum of 1-bromobutane verified the formation of the alkyl halide.
The primary alcohol was protonated by sulfuric acid in the presence of a nucleophile. Sulfuric acid also worked as a catalyst and hastened the rate of the reaction. The protonated OH group exited the reaction as a weakly alkaline water molecule instead of leaving as a basic hydroxide ion. Given that butanol was the primary alcohol, the protonated OH group was effortlessly displaced by the nucleophile in the reaction.
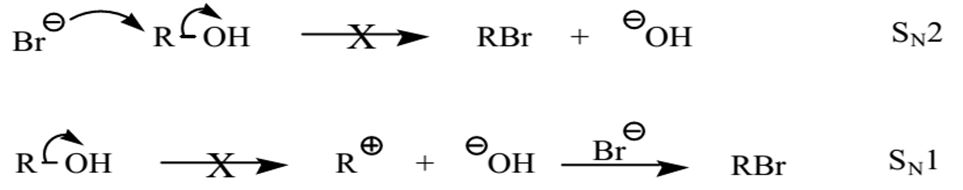
SN1 mechanisms would have occurred if the alcohol were tertiary because the protonated hydroxyl-carbon linkage would break to produce a carbocation via SN1 machinery. The carbocation would then react with the nucleophile to generate a substitution product.
In secondary alcohols, nucleophiles would take the place of the protonated hydroxyl through either SN2 or SN1 mechanisms. The choice of the precise mechanism would be influenced by the detailed conformation of the alkyl group. Hence, secondary and tertiary alcohols readily reacted with concentrated hydrohalic acids such as hydrochloric acid and hydrobromic acid.
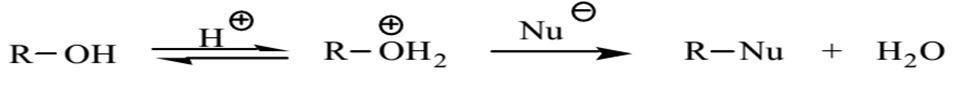
The sulfuric acid served two important purposes in the experiment. The first role was to increase the quantity of protonated alcohols present in the reaction mixture while the second role was to help bind the water molecules produced in the reaction hence changing the equilibrium to favor the formation of the alkyl bromide.
The gas trap was used to confine poisonous gases that were produced during the reaction of sulfuric acid and the reaction mixture during refluxing. The heating process liberated some fumes, which were removed from the round bottom flask with the aid of an aspirator. Flowing tap water was forced through a smooth hose leading to a reduction in the atmospheric pressure of the hose pipe. The reduced pressure allowed the flow of air from the flask containing the toxic fumes to be easily removed.
Refluxing was carried out to accelerate the chemical reaction without the evaporation of the reactants or explosions. The cooling of the vapor before it left the vessel enabled the sustenance of a constant volume. Refluxing was done at 100oC because that was the boiling point of water. At that temperature, the water evaporated thereby producing steam that was used to keep the volume of the reactants constant.
Distillation aimed to separate water from bromobutane. After distillation, the organic layer, which comprised the product was found at the bottom of the vessel because its density was higher than that of the aqueous, inorganic layer. After separating the organic layer from the aqueous layer, it was necessary to add deionized water to the flask to dissolve any unreacted butanol. Sodium bicarbonate was added to the product to react with any remaining sulfuric acid and hydrobromic acid as shown in the below reactions.
Na2CO3 (s) + H2SO4 (aq) → Na2SO4 (aq) + H2O (l) + CO2 (g)
Na2CO3 (s) + 2HBr (aq) → 2NaBr (aq) + H2O (l) + CO2 (g)
In the last steps, it was important to wash the product with deionized water to remove any unreacted sodium bicarbonate into the aqueous layer and get rid of any polar contaminating chemical substances such as butanol that was remaining in the product. Anhydrous sodium sulfate was used as the drying agent to get rid of any water remnants in the final product, which was present in the organic layer.
Sources of Errors
The use of sulfuric acid had certain shortcomings. Sulfuric acid had the propensity to cause side reactions such as the dehydration of alcohol to form an alkene or ether formation. These unwanted products were formed by using up part of the alcohol that was required to form 1-bromobutanol hence thus reducing the output of the alkyl halide.
During distillation, there was a possibility that the distillation setup was not as tight as it should have been hence causing small quantities of the flask contents to escape as a vapor to the surroundings. The components of the escaping vapor included bromobutane hence contributing to the low yield. During the separation of the organic and inorganic phases, it was likely that some amount of the product remained at the interphase of the two layers and was discarded together with the waste.
It was also possible that the washing step introduced errors. There was a chance that part of the product remained in the aqueous phase hence leading to a decrease in the overall yield. Other potential causes of error included the incomplete reaction of the reactants and the loss of product during the transfer of the final product to the sample bottle.
Data Sheet for Substitution Reactions – Reaction Rate Studies
Questions
- In the study of the leaving group, identify the compound that reacted faster.
2-Bromo-2-methylpropane reacted faster than the other compounds. - Based on the answer provided above, which is the better leaving group, Br– or Cl–?
Br is a better leaving group than Cl. - In the study of the alkyl structure, which compound reacted faster?
2-bromo-2-methylpropane - Does the above answer agree with the theory about the stability of the intermediate carbocation? Explain your answer.
The above answer agrees with the theory about the stability of intermediate carbocations. Carbocations become stable with the help of the nearby lone pairs of electrons. Chlorine tends to draw electrons to itself more than bromine. Therefore, Br is more unstable than Cl, which makes it a better leaving group. - Based on the percentages of isopropyl alcohol and water used in making the 40% isopropyl alcohol in water or 60% isopropyl alcohol in water solutions, which is more polar? Explain your answer.
40% isopropyl alcohol exhibits more polarity than 60% alcohol. From the computations in the pre-lab tests, the amount of water added during the preparation of the 40% isopropyl alcohol is greater (12 ml) than the volume of water used in the preparation of 60% isopropyl alcohol (8 ml). Water is also a polar solvent. As a result, 40% isopropyl alcohol is more polar because the extra water contributed to the increase in its polarity. - Does your data from the solvent polarity study support the theory that SN1 reactions proceed faster in a more polar solvent? Explain your answer.
The data support the theory that SN1 reactions proceed faster in a more polar solvent. The reaction involving 40% 2-propanol, which was more polar proceeded much faster than the reactions involving 50% 2-propanol and 60% 2-propanol that were less polar. The observations were attributed to the polarity of the leaving group. Polar solvents enhanced the ability of the nucleophile.
Conclusion
1-Butanol reacted with hydrobromic acid to produce 1-bromobutane and water via an SN2 mechanism. The presence of concentrated sulfuric acid made the hydroxyl group a better leaving group in the form of a hydronium ion, which made the reaction possible. The separation of the water and bromobutane was achieved by distillation because of the difference in their boiling points. The analysis of the IR spectra confirmed the identity of bromobutane by revealing the presence of hydroxyl functional groups in the reactants and their absence in the product.
Additionally, the C-Br bond was evident in the products and absent in the reactants. The experimental yield was only 5.49%, which depicted a high rate of error in the experiment. These errors were partly accountable for the side reactions caused by concentrated sulfuric acid hence leading to the loss of the expected product.
Incomplete reaction and product wastage through experimental processes such as washing, and weighing were also responsible for the low experimental yield. Such errors could be minimized by lengthening the distillation process to guarantee the recovery of most of the product. The washing and weighing processes should be carried out with utmost care to minimize product loss. It was concluded that primary alcohols formed alkyl-halides via SN2 reaction mechanisms.
References
Bruckner, Reinhard. Organic Mechanisms: Reactions, Stereochemistry and Synthesis. Berlin: Springer Science & Business Media, 2010. Print.
Chemical Book. 1-Bromobutane. 2015. Web.
Chemical Book. Sodium Bicarbonate. 2015. Web.
Chemical Book. Sodium Sulfate. 2015. Web.
Chemicalland21. Hydrobromic Acid (Hydrogen Bromide Solution).2015. Web.
National Pollutants Inventory. Sulfuric Acid. 2015. Web.
Okuyama, Tadashi and Howard Maskill. Organic Chemistry: A Mechanistic Approach, Oxford: Oxford University Press, 2013. Print.
PubChem. 1-Butanol. 2015. Web.
Smith, Michael B. Organic Chemistry: An Acid-Base Approach, New York: CRC Press, 2010. Print.
Straumanis, Andrei. Organic Chemistry: A Guided Inquiry for Recitation, Volume 1, Belmont, CA: Brooks Cole, 2011. Print.