The endodermal development, which is the basis of elucidating pancreatic beta cell development, is critical in the treatment of diabetes. The cellular and molecular mechanisms of signaling pathways indicate that exogenous and endogenous coupled with genetic expression regulate endodermal development. DE differentiation into pancreatic progenitors is under the regulation of Nodal, WNT, BMP, and FGF pathways. The development of endocrine cells from DE involves transcription factor-mediated NOTCH, HEDGEHOG, and EGF signaling pathways. The signaling pathway involved in the regulation of pancreatic β cell development entails transcriptional modulation of genes involved in pancreas development and differentiation factors. Activin A and FGF initiate differentiation of hESCs into mesoderm, and further unite to activate a pathway made up of Oct4, Nanog, and Sox2. NODAL pathway, activated by Activin A, leads to the production of SOX 17, FOXA2, PDX1, and NK6X.1 transcriptional factors that regulate pancreatic development.
BMP inactivation leads to differentiation of hESCs into pancreatic progenitors, with PDX1 further directing differentiation into endocrine progenitors. NK6X.1 is responsible for β cell maturation and functionality, as PAX inhibits ARK and glucagon, increasing insulin production. Diabetes mellitus results, due to the failure of the beta cells of the pancreas to function properly. Empirical evidence focusing on mice deficient of FoxO1, which mediates β cell proliferation and functioning, reveals that β cells’ inability to regenerate causes hyperglycemia and diabetes.
Introduction
The development of pancreatic beta cells follows the pattern of endoderm formation during embryonic development, forming the basis for developing cell-based therapies. The induction of pluripotent stem cells (PSCs) such as mouse embryonic stem cells (mESCs), human embryonic stem cells (hESCs), and induced pluripotent stem cells (iPSCs) make them differentiate into pancreatic beta cells. The differentiation mechanism of PSCs is subject to signaling pathways and genetic expression. Signaling pathways, such as Nodal, FGF, WNT, and BMP, regulate the differentiation of pancreatic cells and the development of endocrine cells. A close review of the literature elucidates the mechanism by which PSCs differentiate into functional pancreatic beta cells.
Understanding the Signaling Pathways Regulating the Development of the Endoderm
Endodermal formation during embryonic development
Endodermal formation is a complex cellular and molecular process that occurs during embryonic development. Primarily, it involves the differentiation of PSCs (mESCs and hESCs) into three germ layers: the ectoderm, mesoderm, and endoderm. Essentially, endodermal formation entails the process of gastrulation, followed by the differentiation of this layer to generate visceral organs in a process called organogenesis. In the formation of the endoderm, the blastula undergoes a series of morphogenic movements. The first of these movements is invagination, in which the outer layer moves inward into the blastula to form a primitive streak (PS). The subsequent morphogenic movement is the involution of the invaginated outer layer, which results in the formation of the inner and outer layers of the blastula. The ingression of the epithelial cells, the third morphogenic movement, leads to the formation of mesenchymal cells. Epiboly is the final morphogenetic movement, resulting in endoderm formation through the reorganization and distribution of inner cell mass within the blastocyst. The differentiation of the endoderm leads to the generation of internal organs such as lungs, heart, kidneys, pancreas, liver, and spleen.
Signaling pathways regulating endodermal development
The endodermal development is subject to the control of diverse signaling pathways. The established signaling pathways that control endodermal development are Nodal/Activin A, FGF, WNT, and BMP. The Nodal/Activin A signaling pathway controls the formation of endoderm and mesoderm in embryonic development. Evidently, in both in vitro and in vivo conditions, Nodal/Activin A induces the formation of both the posterior PS and anterior PS. The concentration of Activin A influences PS formation, as high concentration stimulate the formation of the anterior PS while low concentration induces the formation of the posterior PS. The FGF signaling pathway controls the differentiation of endodermal cells. Fundamentally, the FGF pathway suppresses the expression of pluripotency markers in mESCs.
The obstruction of this pathway implies that PSCs cannot differentiate into specific cells. The WNT signaling pathway regulates endoderm formation by inducing PS formation. The PS formation enhances differentiation of endoderm, because it promotes the formation of the inner cell mass. Unlike Nodal, WNT only induces the formation of PS at the posterior end. Just like other signaling pathways, BMP controls differentiation in endodermal development. BMP induces the formation of PS within populations that express T. In essence, BMP works with Activin A and LIF in promoting differentiation of cells during endodermal development.
Genes required for endoderm specification
Endodermal development is also subject to the control of genetic expression. The expression of SOX17 and Mixl1 individually and collectively promotes endodermal differentiation and the formation of definitive endodermal (DE). The expression of SOX17 and Mixl1 genes has a synergistic effect on the formation of PS and DE in the WNT signaling pathway. In both humans and mice, expression of Oct4, SOX2, and Nanog specify the self-renewal and pluripotency of mESCs and hESCs. Moreover, the expression of FOXA2 and SOX17 has a synergistic effect on the formation of PS and DE. Evidently, hESCs that express FOXA2 and SOX17 specify mesoderm and mesendoderm in the BMP4 signaling pathway. FGF2, WNT, and Activn A signaling pathways control the core transcriptional network in hESCs, while LIF, WNT, and BMP4 signaling pathways control the core transcriptional network in mESCs, as illustrated in Figures 1 and 2 below.
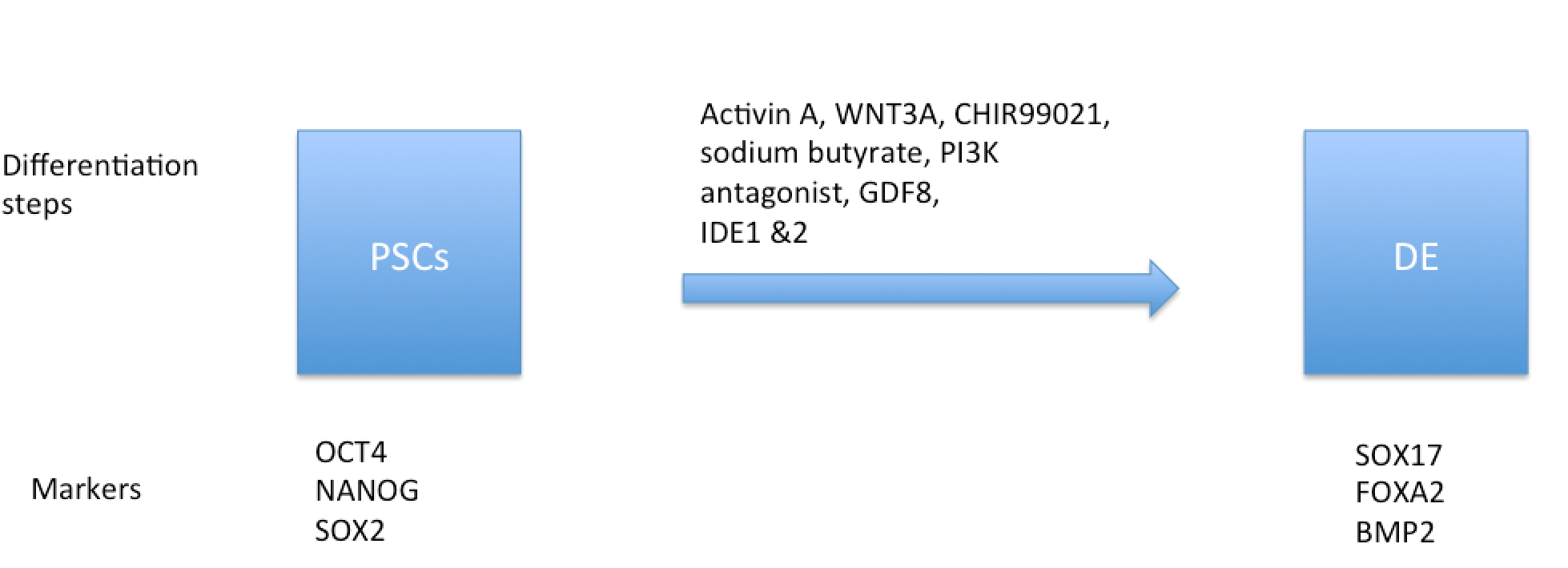
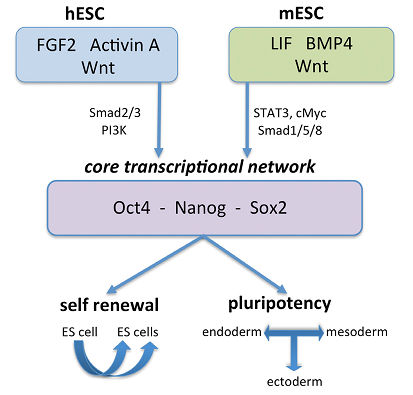
Differentiation of stem cells into the endoderm
PSCs differentiate into the endoderm when induced. Manipulation of the culture conditions and of the expression of specific regulatory genes in stem cells activates signaling pathways that allow differentiation and eventually lead to endodermal development. The induction of PSCs differentiates them into specialized lineages. WNT and Nodal are the primary inducers of differentiation, as they induce the formation of the PS and DE. Furthermore, Activin A is an exogenous inducer, which enhances the induction effect of the endogenous inducers. The induction of hESCs with CHIR99021 and wortmannin (PI3K inhibitor) boosts the differentiation of SOX17-positive cells by 90%. The addition of growth factors into the stem cell cultures promotes their differentiation into the endoderm.
Signaling Pathways Regulating the Development of Pancreatic Progenitors
Multipotent pancreatic progenitor cells (MPCs) originate from the DE and generate the exocrine cells that differentiate into insulin-secreting β cells via transcription and signaling pathways. The induction of DE differentiation from hiPSCs and hESCs involves the Nodal, WNT, BMP, and FGF pathways. Nodal/Activin A, a TGF-beta growth factor, has been shown to stimulate PS differentiation into the DE in vertebrates. In vitro experiments have shown that an elevated Activin A dosage (>10ng/mg) signals the formation of the anterior PS and the subsequent DE from adherent ESC cultures. However, a DE marker called Gsc is lowly expressed in embryoid bodies (EBs) cultured in a serum-free monolayer after Activin A treatment. Therefore, Activin A induction appears to signal ED development via factors contained in serum.
Similarly, the WNT signaling pathway mediates the formation of the posterior PS in serum-free mESC cultures. Evidence from in vitro studies indicates that the initiation of the PS formation in EBs and vertebrate ESCs is induced through the WNT pathway. WTN induces SOX17 and Mixl1 expression in the presence of Activin A. The suppression of WNT signals has been associated with inhibited PS and DE output, indicating that this pathway mediates the development of the posterior PS but not the anterior lineages.
In the BMP pathway, neural differentiation is inhibited by BMP4, a TGF-beta growth factor, in the presence of LIF (a signaling factor), which maintains ESCs in a pluripotent state. When LIF is low, BMP4 stimulates the development of mESCs into ED progenitor cells. In vitro studies have established that BMP4 can stimulate the formation of ectoderm and trophoblast cells from adherent ESC cultures; hence, BMP4 is a signaling factor in the PS formation.
Unlike Activin A, WNT, and BMP4, the FGF pathway does not directly initiate DE differentiation. Instead, FGF ligands inhibit the spontaneous ESC differentiation required for ectodermal and mesodermal commitment to form ED. In this way, the pathway controls PS and mesoderm cell lineages through a regulated expression of pluripotency factors.
Signaling Pathways Regulating the Development of Pancreatic Endocrine Cells
In humans, insulin cells are the first pancreatic endocrine cells to develop, followed by glucagon at eight and nine weeks, respectively. The β cell differentiation from DE cells is induced by the controlled expression of NKX2.2, ISL1, and PDX1 transcription factors at different stages. The aim is to control the development of the DE cells into pancreatic endocrine cells.
The differentiation of the DE cells into β cells requires the up-regulation of the NOTCH and HEDGEHOG pathways. After DE formation, the BMP pathway is needed to regulate PDX1 expression to promote further pancreatic development. In vitro experiments have used inhibitors such as KAAD, DAPT, retinoic acid, and cyclopamine to up-regulate PDX1, an important factor in β cell formation. Another factor, fibroblast growth factor 10 (FGF10), induces the NOTCH pathway required to initiate PDX-1 expression in β progenitor cells.
The epidermal growth factor (EGF) is another pathway involved in endocrine cell differentiation. EGF signaling stimulates hESC-derived PDX1 progenitor cells that differentiate into β cells. The addition of the Indolactam V molecule stimulates DE cells derived from ESCs to develop PDX-expressing cells that form β cells. Other molecules that induce PDX1-mediated β cell differentiation from the DE through EGF signaling include reserpine, TVZ, and VMAT2.
Understanding the Signaling Pathways Regulating the Development of Pancreatic Beta Cells
Empirical evidence has shown that differentiated hESCs produce markers including SOX17, FOXA2, CXCR4, and GSC, with the NODAL and WNT signaling pathways triggering DE differentiation. These pathways further unite in the activation of a central transcription network—comprised of Oct4, Nanog, and SOX2—that controls the pluripotency and self-renewal of ESCs. In hESCs, NODAL in particular, which is activated by Activin A (a member of TGFβ), is the main inducer of endodermal development. Furthermore, in vitro studies reveal that Activin A in unison with wortmannin (P13K antagonist) and CHIR99021 increases the population of SOX17 positive cells, while Activin A together with WNT activators such as WNT3A OR CHIR9902 enhances DE differentiation.
BMP signaling inhibition is vital for the differentiation of hESCs into pancreatic progenitors and endocrine cells. The activation of the pathway after pancreatic development is vital for PDX1 maintenance with Indolactam V, which activates protein Kinase C and regulates PDX1 secretin- cells. PDX1 is important in pancreatic development, but it is required only in the later stages of endocrine cell development for enhanced β cell development and function. Additionally, NOTCH and HEDGEHOG signaling pathways control endocrine differentiation, with another transcription factor neurogenin 3 (NOG3) increasing simultaneously with PDX1 progenitor cell production. The inhibition of HEDGEHOG by KAAD cyclopamines activates overproduction of PDX1-producing cells, while NOTCH activation by fibroblast growth factor 10 heightens the proliferation of PDX1-producing pancreatic precursors. The inhibition of NOTCH by DAPT leads to the production of Homeobox protein NK -6 homolog (NK6X.1), which is vital to pancreatic cell development and the generation of functional mature beta cells. Overexpression of another transcription factor, PAX 4, inhibits ARK and glucagon, leading to increased insulin production. Figure 3 below illustrates the development of pancreatic β cells.
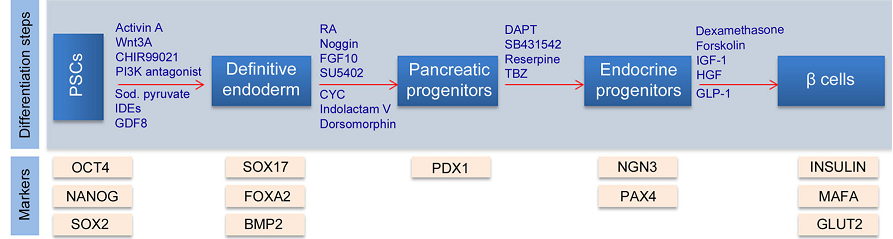
Understanding Beta Cell Failure in Diabetes Mellitus
The autoimmune destruction of β cells is responsible for type 1 diabetes mellitus, while type 2 arises either from β cells’ insufficient production of insulin or from a total non-response of cells with insulin receptors to insulin. Beta cell failure may be caused by myriad factors, including increased levels of reactive nitrogen species and metabolic stress. In metabolic stress, autophagy transforms into a pathologic process due to superfluous nutrients. The stimulation of unfolded protein reply affects insulin production, and this inability to metabolize glucose may result in the production of reactive oxygen species, which in turn severs glucose sensing from insulin production. Ordinarily, pancreatic β cells respond to increased cell demand for glucose by increasing insulin production, generating new beta cells from precursor cells, and proliferating existing beta cells. The cells’ inability to dedifferentiate in diabetes has been empirically determined to be due to transcription factor FOX01, which integrates β cell proliferation and function, in addition to directing the cell fates of differentiating endocrine cells. In vitro studies of FOX01-deficient murines reveal that β cells were unable to dedifferentiate, underscoring FOX01’s role in the regression of beta cells to ancestral cells innately capable of producing Nanog, Oct4, Neurogenin, and L-Myc.
Conclusion
Endodermal development in embryos is a complex process that is subject to diverse regulatory mechanisms and signaling pathways in the cell. A review of the literature reveals that the Nodal, WNT, BMP, and FGF signaling pathways control endodermal development and differentiation in mESCs and hESCs. Moreover, SOX17, Mixi1, Oct4, SOX2, and Nanog are defined genes that dictate the self-renewal and pluripotency of PSCs. The combined effect of these signaling pathways and genetic expression influences the development of pancreatic beta cells.
References
Abdelalim, E., Bonnefond, A., Bennaceur-Griscelli, A., & Froguel, P. (2014). Pluripotent stem cells as a potential too for disease modeling a cell therapy in diabetes. Stem Cell Reviews and Reports, 10(1), 327-337.
Abdelalim, E., & Emara, M. (2015). Advances and challenges in the differentiation of pluripotent stem cells into pancreatic β cells. World Journal of Stem Cells, 7(1), 174-181.
Conrad, E., Stein, R., & Hunter, C. (2014). Revealing transcription factors during human pancreatic β cell development. Trends in Endocrinology & Metabolism, 25(8), 407-414.
Pagliuca, F., Millman, J., Gurtler, M., Segel, M., Van Dervort, A., Ryu, J., Peterson, Q., Greiner, D., & Melton, D. (2014). Generation of functional human pancreatic β cells in vitro. Cell, 159(1), 428-439.
Sui, L., Bouwens, L., & Mfopou, J. (2013). Signaling pathways during maintenance and DE differentiation of embryonic stem cells. International Journal of Developmental Biology, 57(1), 1-12.
Talchai, C., Xuan, S., Lin, H., Sussel, L., & Accili, D. (2012). Pancreatic β-Cell dedifferentiation as mechanism of diabetic β-Cell failure. Cell, 150(6), 1223-1234.