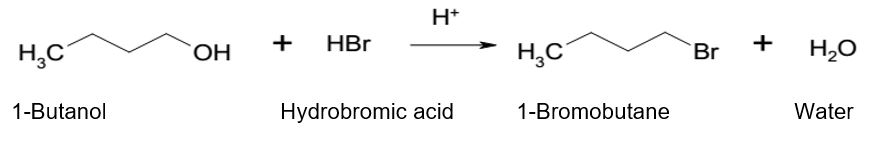
Purpose
The purpose of the experiment is to study substitution reactions of alcohols because they can react as nucleophiles or electrophiles, depending on prevailing conditions of the reaction. This experiment illustrates the reaction of 1-butanol with hydrobromic acid to form 1-bromobutane.
Physical Properties
1-Butanol
State: Colorless liquid
Melting point: -89°C
Boiling point: 117.6°C
Water solubility: 80g/L at 20°C
Density: 0.81g/mL at 25°C
Molecular formula: C4H10O
Molar mass: 74.12g/mol
Hazards: Toxic, harmful, and highly flammable
Hydrobromic acid
State: Colorless liquid
Melting point: -87°C
Boiling point: -67°C
Water solubility: Miscible
Density: 1.49g/mL at 25°C
Molecular formula: HBr
Molar mass: 80.9g/mol
Hazards: Corrosive and irritant
Sulfuric Acid
State: Colorless oily liquid
Melting point: 10°C
Boiling point: 290°C
Water solubility: Miscible
Density: 1.84g/mL at 25°C
Molecular formula: H2SO4
Molar mass: 98.08g/mol
Hazards: Corrosive, highly flammable, corrosive, and irritant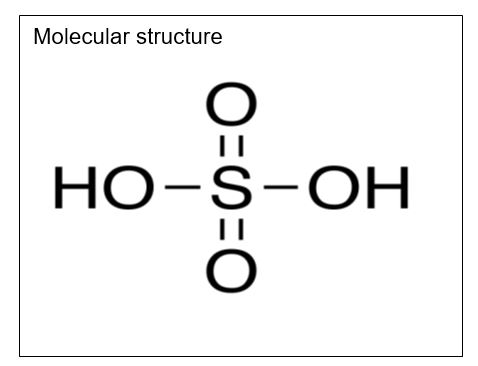
Sodium Bicarbonate
State: White crystals or powder
Molecular formula: NaHCO3
Molar mass: 84.01g/mol
Melting point: 300°C
Boiling point: 851°C
Water solubility: 90g/L at 20°C
Density: 2.16g/mL
Hazards: Irritant, and toxic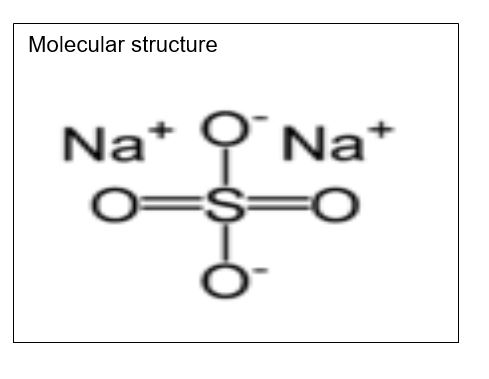
Sodium Sulfate
State: White crystals or powder
Melting point: 884°C
Boiling point: 1700°C
Water solubility: 18mg/L at 20°C
Density: 2.68g/mL at 25°C
Molecular formula: Na2SO4
Molar: 142.04g/mol
Hazards: Irritant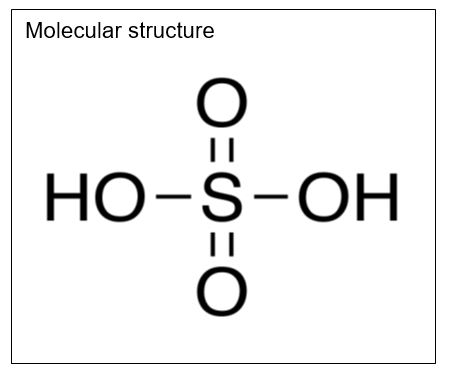
1-Bromobutane
State: Yellow or colorless liquid
Melting point: –112°C
Boiling point: 100-101°C
Water solubility: 0.608g/L at 20°C
Density: 1.276g/mL at 25°C
Molecular formula: C4H9Br
Molar mass: 137.02g/mol
Hazards: Irritant, highly flammable, and dangerous for the environment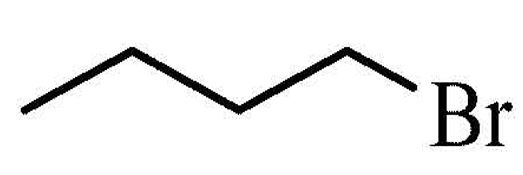
Procedure
To prepare 10-bromobutane, 6.2ml of 1-butanol was measured properly and poured into a 100ml round bottom flask. 10 ml of 48% hydrogen bromide (HBr) and 4ml of concentrated sulfuric acid (H2SO4) were measured and poured into the round bottom flask with 6.2ml 1-butanol gradually while swirling. The reacting contents in the flask were immersed in an ice bath to cool. Two boiling chips were inserted into the flask and the reaction apparatus was set by connecting a reflux condenser to the top of the flask.
Water hoses were linked to the reflux condenser, and a vacuum tube was connected on top of the condenser. The other unconnected end of the vacuum hose was joined to the sink aspirator via the gas trap. The sink aspirator was turned on and adjusted to run at full blast. The suction pressure of the set apparatus was tested by placing a finger on the open joint of the vacuum connecting tube.
After confirming that the apparatus was working properly, the reaction mixture in the flask was then heated at reflux for 45 minutes. When the heating duration of 45 minutes expired, the mixture in the flask was allowed to cool at room temperature. To the cooled mixture, 10ml of de-ionized water was added via the condenser gradually and carefully.
Additional two boiling chips were then added to the mixture, and a vacuum was disconnected from the apparatus to create a simple distillation apparatus. The distillate obtained from the distillation of the mixture was collected using a 25ml round bottom flask that was immersed in the ice bath. When the temperature of the distillate reached 100C, the collection of distillates was stopped.
The aqueous layers were removed from the distillate using a transfer pipette and put in a ‘waste flask’. Three consecutive washes were done using de-ionized water and sodium bicarbonate to separate the organic phase from the inorganic phase. The first, the second, and the third washes used 5ml of de-ionized, 5ml of 5% sodium bicarbonate, and 5ml of de-ionized water, which were was added to the organic layer and the aqueous layers separated and combined with the collected aqueous layer.
The content of the organic layer was dried by placing anhydrous sodium sulfate over the layer. The dried product of the organic layer was then decanted into a pre-weighed sample bottle. The results of the experiment were obtained by weighing the organic product in the sample bottle, running IR, and calculating percent yield.
Results
Theoretical yield
Mass of 1-butanol= 0.81 g/mL * 6.2 mL = 5.022g
Moles of 1-butanol= 5.022g / 74.12 g/mol = 0.0677 mol
Theoretical yield = (0.0677 mol *137.02 g/mol) / 1 mol of 1-bromobutane= 9.28g
Percent yield
Empty sample bottle= 14.97 g
Sample bottle+ product= 15.48 g
Mass of the product= 15.48g – 14.97g= 0.51g
Percent yield= (Actual yield/ theoretical yield) * 100
(0.51g/9.28g) *100= 5.49 %
Synopsis of the Results
The results show that there is a great difference between the theoretical yield and the actual yield of 1-bromobutane. Calculation of the theoretical yield shows that the experiment should have yielded 9.28g of 1-bromobutane from 5.022g of 1-butanol. However, the experiment indicates that the actual yield of 1-brombutane from 5.022g is 0.51g. Thus, the percent yield of 1-bromobutane is 5.49% of the theoretical yield. The low yield of 1-bromobutane indicates that some errors within the experimental process affected the yields.
The first possible reason is that the heating of reactants led to the loss of 1-bromobutane through evaporation. The second reason is that the distillation and condensation processes could have led to the loss of 1-bromobutane because it is a volatile liquid. The third reason is that washing of the 1-bromobutane with de-ionized water and sodium bicarbonate contributed to the loss of 1-bromobutane through the removal of aqueous layers. The fifth reason is that some 1-butanol did not react with hydrobromic acid.
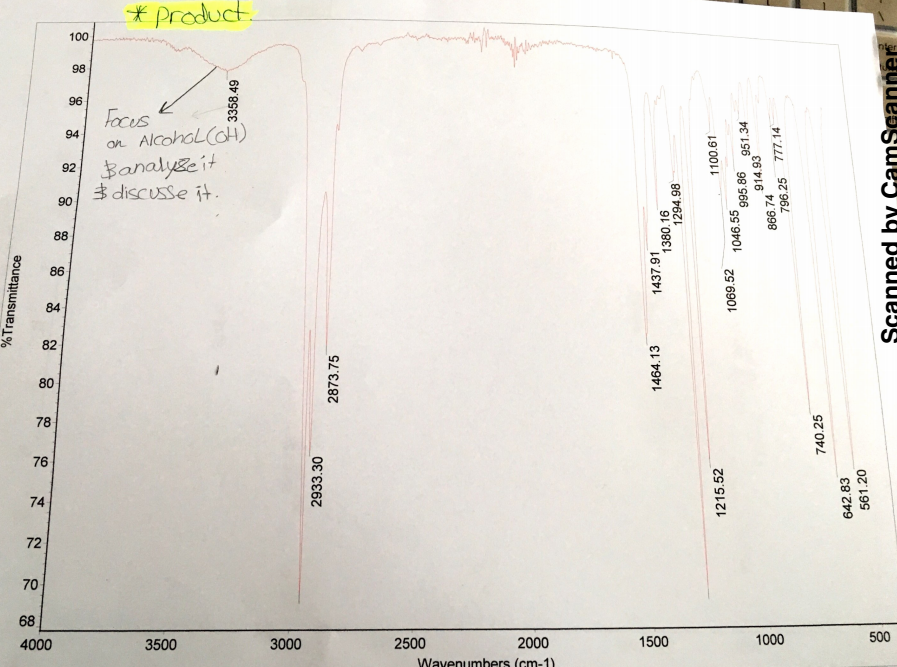
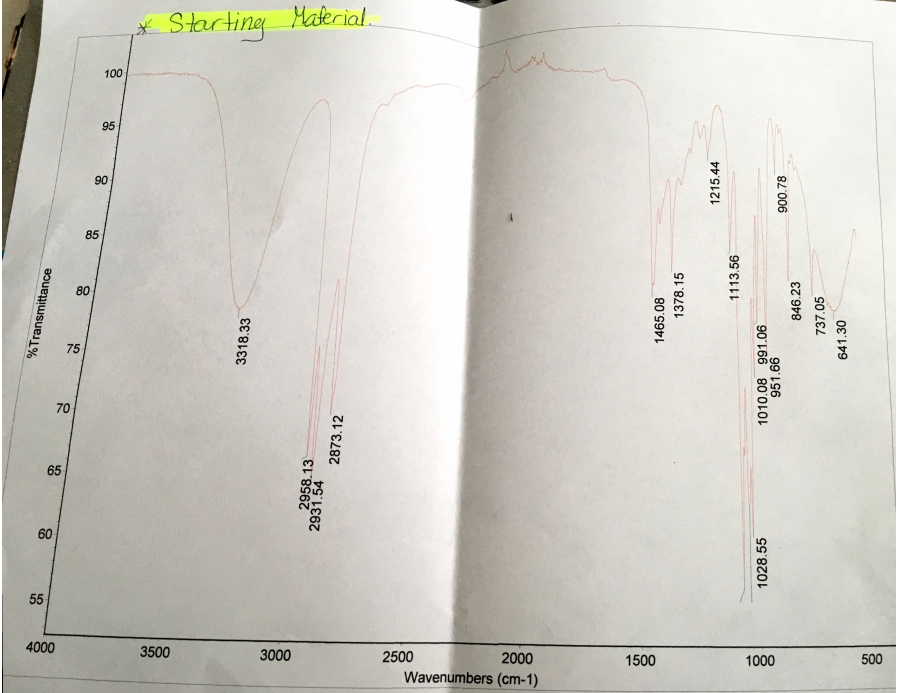
Analysis of the IR spectra shows the existence of the O-H troughs before and after the reaction within the range of 3200cm-1 and 3500cm-2 wavenumbers. Before the reaction, the IR spectrum had a broad O-H trough peaking at 3318.33cm-1 with a transmittance of approximately 80%. After the experiment, the IR spectrum had a shallow O-H trough peaking at 3358.49cm-1 with a transmittance of approximately 98%. Hence, the existence of a shallow trough and peak within the range of the O-H trough indicates that the products had some 1-butanol that did not react.
Discussion
A reaction that entails the displacement of a functional group by another functional group is called a substitution reaction. Two types of substitution reactions that exist are electrophilic substitution and nucleophilic substitution. Electrophilic substitution is a type of reaction where a functional group tends to accept electrons from another compound while nucleophilic substitution is a type of reaction where a functional group tends to donate electrons to another compound. In this experiment, the type of substitution is nucleophilic substitution because it entails the reaction of 1-butanol and hydrobromic acid.
The mechanisms of nucleophilic substitution can either be unimolecular (SN1) or bimolecular (SN2). The mechanism of nucleophilic substitution in this experiment is the SN2 reaction because 1-butanol is a primary alcohol, bromine ion is a strong nucleophile, and the reaction occurs in a polar aprotic solvent. The SN2 reaction has no intermediate carbocation because its primary carbocation is quite unstable. In the SN1 reaction, there is the formation of intermediate because its occurrence requires tertiary carbocation that is very stable.
In this, the study illustrated the reaction of 1-butanol that lead to the formation of 1-bromobutane. The reaction is a nucleophilic substitution, which follows the SN2 reaction mechanism. The functional group of 1-butanol is (-OH) while the functional group of hydrobromic acid is bromide ion (Br–). For the nucleophilic substitution to happen, hydrogen ions (H+) from sulfuric acid protonates -OH of 1-butanol and forms oxonium ions (-OH2+).
In this view, -OH acts as an electron donor by donating the lone pair of electrons to H+, hence, stabilizing it. The formation of the oxonium ions creates a leaving group that bromide ions can easily displace. As bromide ion is a strong nucleophile, it attacks the primary carbon of 1-butanol and displaces oxonium ion, which is a favorable leaving group, in the form of a neutral water molecule. Thus, protonation of 1-butanol catalyzed the nucleophilic substitution of -OH with Br– resulting in the formation of 1-bromobutane.
The reagents in the round bottom flask were heated at reflux for 45 minutes. Heating at reflux was necessary because sulfuric acid, hydrobromic acid, and 1-butanol are volatile reagents that easily evaporate when left in the air or heated. Heating at reflux entails heating reactants in a sealed system where no vapor can escape. The reflux system comprises a boiling tube and condenser. When reactants vaporize in the boiling tube, they move into the condenser where they condense and flow back into the boiling tube without any loss. In the experiment, heating at reflux prevented the loss of reactants in the form of vapor from the round bottom flask. To distill the reactants in the round bottom flask, the reflux system was converted into a simple distillation apparatus.
The apparatus for distilling 1-bromobutane comprised the gas trap, which was connected at the end of the vacuum hose. Since the heating process of the mixture generated noxious fumes of sulfuric acid, hydrobromic acid, and1-butanol, the gas trap prevented them from escaping into the air. Fundamentally, the gas trap protects experimenters in the laboratory from the exposure of harmful gases and prevents environmental pollution.
The distillation process aimed at separating 1-bromobutanol from the mixture of organic and inorganic phases in the round bottom flask. Given that 1-bromobutane boils at 100°C, the distillation process was stopped at 100°C. Distillation of reactants at temperatures above 100°C would cause evaporation of other compounds such as 1-butanol because its boiling point is 117°C. Therefore, the threshold of 100°C was critical in maintaining the purity of 1-bromobutane distilled from the mixture.
The distillate collected had the organic layer and the inorganic layer. The organic layer formed the upper layer while the inorganic layer formed the lower layer. 1-bromobutane, which is the product of the nucleophilic substitution, formed the upper layer because is it less dense than the inorganic layer. The density of 1-bromobutane is 1.276g/mL while the density for the aqueous layer is greater than this density for it contains salts with high densities. De-ionized water was added to the organic layer to wash acid residues and other charged ions. Subsequently, sodium bicarbonate was added to the organic layer to extract the organic phase from the inorganic phase, neutralize acids, and make the aqueous solution basic. The de-ionized water was added once more to remove residues of sodium bicarbonate previously added. Anhydrous sodium sulfate was a drying agent that removed water molecules from 1-bromobutane.
The experiment managed to accomplish its purpose of illustrating the formation of 1-bromobutane from the reaction of 1-butanol and hydrobromic acid in acidified conditions. The results illustrate that theoretical yield and actual yield of 1-bromobutane differ considerably. Theoretically, the calculation of yield indicates that the experiment ought to have yielded 9.28g of 1-bromobutane from 5.022g of 1-butanol. Nevertheless, the experiment found the actual yield of 1-brombutane from 5.022g 1-butal to be 0.51g.
Calculation of percent yield shows that 0.51g of 1-bromobutane is 5.49% of the theoretical yield. The low yield points out that there are major sources of errors that affected the yields. The evaporation of 1-butanol and 1-bromobutane during distillation is a possible source of the error. Washing of the 1-bromobutane with de-ionized water and sodium bicarbonate is a possible source of error because it removed some 1-bromobutane. Another possible source of error is that some 1-butanol did not react with hydrobromic acid.
The IR spectrum tests if all 1-butanol molecules reacted with hydrobromic acid in the acidic condition to form 1-bromobutane. Essentially, the IR spectrum indicates the presence of -OH in both reactants and products. Critical analysis of the IR spectra generated before and after reaction depicts the presence of the O-H troughs within the range of 3200cm-1 and 3500cm-2 wavenumbers. The IR spectrum generated before the reaction exhibits a broad O-H trough that peaks at 3318.33cm-1 with a transmittance of approximately 80% (See Appendix A).
The IR spectrum generated after the experiment has a shallow O-H trough that peaks at 3358.49cm-1 with a transmittance of approximately 98%. The expectation was that the spectrum generated after the reaction should not have the O-H trough within the wavenumbers in the range of 3200cm-1 and 3500cm-2 because all molecules of 1-butanol should have reacted (See Appendix B). Therefore, the existence of a shallow trough and peak within the range of the O-H trough shows that some molecules of 1-butanol did not react with hydrobromic acid to form 1-bromobutane.
References
Chemical Book: 1-bromobutane 2008. Web.
Chemical Book: 1-butanol 2008. Web.
Chemical Book: Hydrogen Bromide 2008. Web.
Chemical Book: Sodium bicarbonate 2008. Web.
Chemical Book: Sodium sulfate 2008. Web.
Chemical Book: Sulfuric acid 2008. Web.
Questions
- The compound that reacted faster is 2-bromo-2-methylpropane for it the reaction happened in 53 seconds.
- The better leaving group is Br– because it is more electronegative when compared to Cl–, thus, it does not bind to carbon atom tightly.
- The compound that reacted faster is 2-bromo-2-methylpropane since it occurred within the period of 47 seconds.
- The stated answer above does not agree with the theory of the stability the intermediate carbocation because water molecules can easily attack the most stable carbocations, which is different from the reactivity of alkyl halides with other nucleophiles.
- The more polar solution is the one that has a lot of water in it because water is a polar solvent that increases the polarity of solutions. In this case, the solution with 40% isopropyl alcohol is a more polar solution than the solution with 60% isopropyl alcohol.
- The data collected do support the theory that SN1 reactions proceed faster in a more polar solvent. Critical analysis of data indicates that reactivity of propanol decreases its percent increases. From the data, the solution with 40%, 50%, and 60% of propanol took 25 seconds, 47 seconds, and 101 seconds correspondingly for reactions to take place.
Appendices
Appendix A: Spectrum of starting material
Appendix B: Spectrum of product